Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (4): 250-263.doi: 10.12336/biomatertransl.2022.04.005
• REVIEW • Previous Articles Next Articles
Jingyu Fan1, Elizabeth Pung1, Yuan Lin2, Qian Wang1,*( )
)
Received:2022-11-09
Revised:2022-12-09
Accepted:2022-12-20
Online:2022-12-29
Published:2022-12-28
Contact:
Qian Wang
E-mail:Wang263@mailbox.sc.edu
About author:Qian Wang,Wang263@mailbox.sc.edu.
Fan, J.; Pung, E.; Lin, Y.; Wang, Q. Recent development of hydrogen sulfide-releasing biomaterials as novel therapies: a narrative review. Biomater Transl. 2022, 3(4), 250-263.
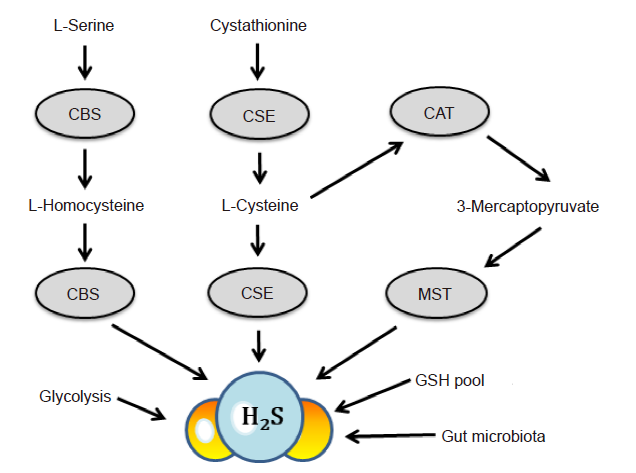
Figure 1. Biosynthesis and catabolism of H2S. In mammals, H2S is produced endogenously from cysteine, serine, homocysteine, and other substrates primarily through the actions of three major enzymes. CBS is mainly localized in the nervous system, brain and liver; CSE is mainly localized in the cardiovascular system to produce H2S; MST is predominantly localized in mitochondria. In addition, the activities of gut microbiota, glycolysis and phosphogluconate of glucose, the GSH and “sulfane sulfur” pools may also contribute to the maintenance of H2S concentrations in plasma and tissue.11,28?-30 CAT: cystine aminotransferase; CBS: cystathionine–β–synthase; CSE: cystathionine–γ–lyase; GSH; glutathione; H2S: hydrogen sulfide; MST: mercaptopyruvate sulfurtransferase.
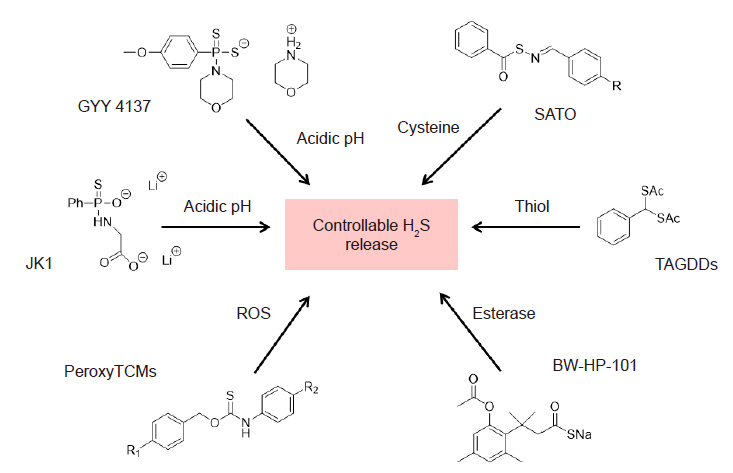
Figure 2. Donor compounds for H2S release. Recent advances in the development of H2S donors has revealed multiple types of donors, namely, pH–sensitive donors including JK1 and GYY 4137,43–45 enzyme–activated donors such as BW–HP–101,46–48 reactive–oxygen species such as PeroxyTCMs,49 and thiol–triggered donors including TAGDDs and SATO.50,51 H2S: hydrogen sulfide; TAGDD: thiol–activated gem–dithiol–based H2S donor; SATO: S–aroylthiooxime; PeroxyTCM: PeroxyThioCarbaMate.
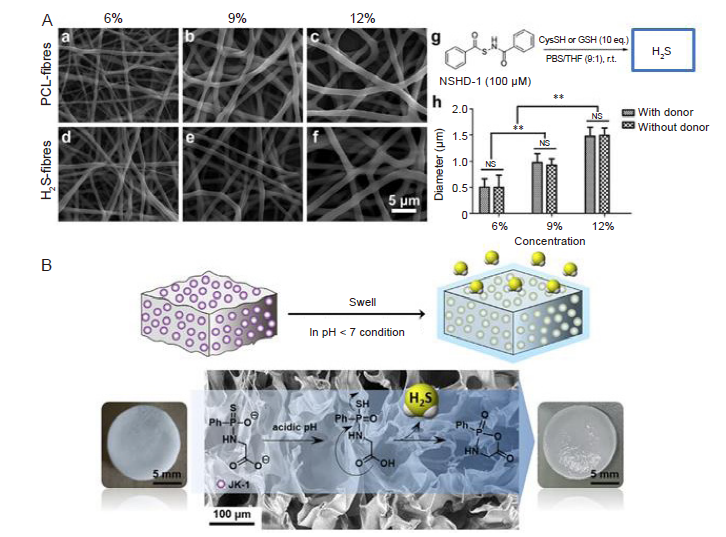
Figure 3. Physical incorporation of H2S donor into biomaterials. (A) H2S–release fibres by incorporating thiol–dependent H2S donor, NSHD–1, in the electrospun PCL–fibres: SEM images of H2S–fibres (a–c) and PCL–fibres (d–f). All images share the same scale bar (5 μm) in f. (g) The H2S donor, NSHD–1, can release H2S in the presence of cysteine or GSH. (h) Fibre diameters plot as a function of solution concentrations. The dopant, NSHD1, has no obvious effect on fibre diameters.65 (B) H2S–releasing sponge sodium alginate/JK–1 by incorporating the pH–dependent H2S donor JK–1 into an alginate sponge obtained by crosslinking sodium alginate with Ca2+.57 Reprinted from Feng et al.65 and Zhao et al.57 Copyright 2015 and 2020, with permission from Elsevier Ltd. GSH: glutathione; NSHD–1: N–(benzoylthio) benzamide; PCL: polycaprolactone; SEM: scanning electron microscope.
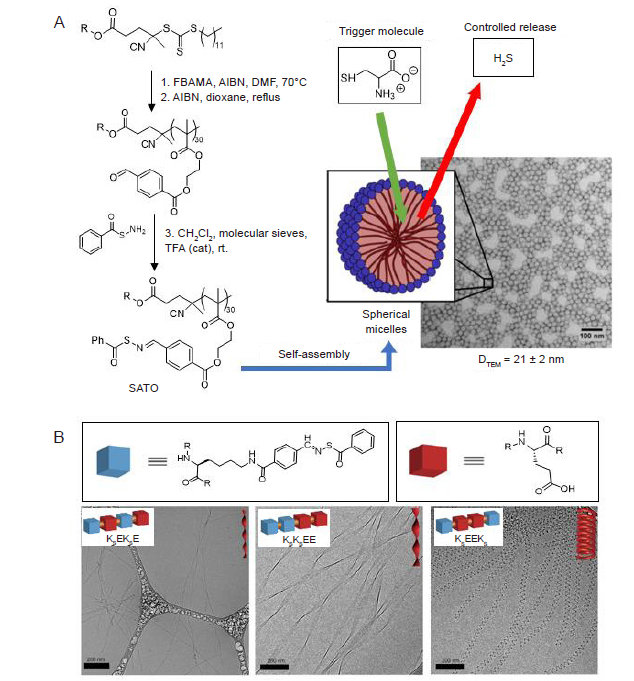
Figure 4. H2S donor units covalently linked to material backbones. (A) H2S–releasing SATO–unit was incorporated to the polymer, which could be self–assembled into spherical micelles with an average diameter of 21 ± 2 nm. Reprinted from Foster et al.72 (B) Three isomeric peptide?H2S donor conjugates assembled into twisted ribbons and nanocoils in aqueous solution. Reprinted from Wang et al.74 AIBN: 2,2′–azobis(2–methylpropionitrile; DMF: dimethylformamide; FBEMA: 2–(4–formylbenzoyloxy)ethyl methacrylate; H2S: hydrogen sulfide; rt: room temperature; SATO: S–aroylthiooxime; SEM: scanning electron microscope; TFA: trifluoroacetic acid.
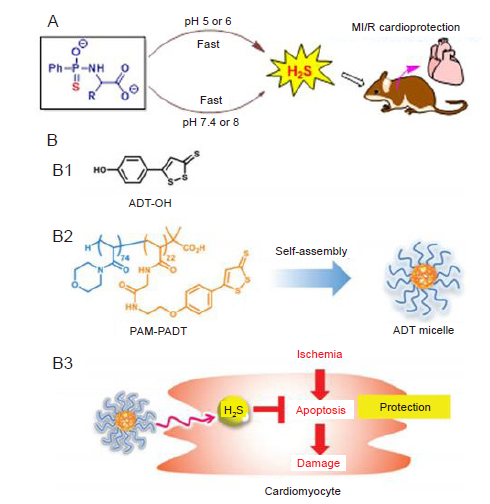
Figure 5. H2S donors act in cardiovascular disease. (A) JK1 gives rise to faster H2S release under acidic condition, of which is distinctive feature of ischemia microenvironment compared to normal physiological condition. Reprinted with permission from Kang et al.45 Copyright ? 2016 American Chemical Society. (B) H2S donor micelles protect cardiomyocytes from ischemic cell death. (B1) Chemical structure of ADT–OH. (B2) A block copolymer having ADT–groups (PAM–PADT) forms ADT micelles by self–assembly. (B3) Intracellular release of H2S from ADT micelles prevents apoptotic damage of cardiomyocytes under ischemic condition. Reprinted with permission from Takatani–Nakase et al.85 Copyright ? Royal Society of Chemistry 2017. ADT: anethole dithiolethione; ADT–OH: 5–(4–hydroxyphenyl)–3H–1,2–dithiole–3–thione; H2S: hydrogen sulfide; PAM–PADT: poly (N–acryloy morpholine)–poly anethole dithiolethione.
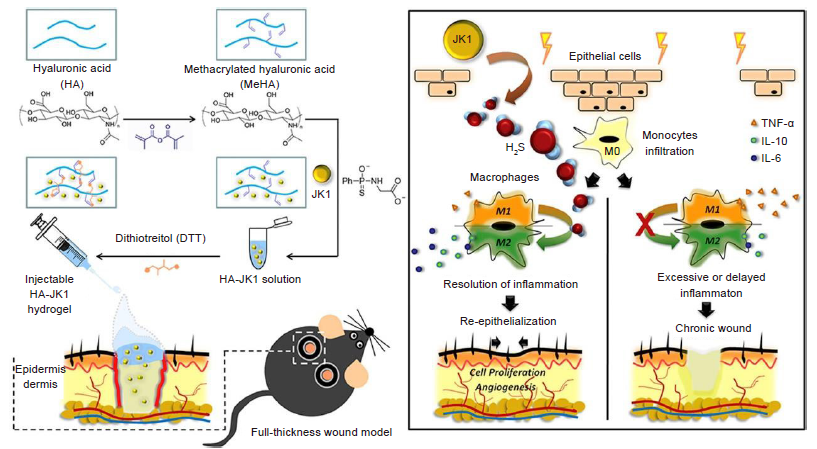
Figure 6. Schematic illustrating the preparation of injectable HA–JK1 hydrogel and its application to full–thickness dermal wound. (Left) JK1, as H2S donor, was incorporated in the HA based injectable hydrogel, which can be used in the mouse wonder model system. (Right) The local low pH condition near the wound could promoted the fast release of H2S of JK1, which could effectively accelerate the wound healing through promoting cell proliferation, angiogenesis and more importantly, suppressing inflammation via inducing M2 macrophage polarization. Reprinted from Wu et al.56 Copyright 2019, with permission from Elsevier Ltd. H2S: hydrogen sulfide; HA: hyaluronic acid; IL: interleukin; TNF–α: tumour necrosis factor–α.
| Application | H2S donor/biomaterials | Research model | Effects/outcome | Proposed mechanism | Reference |
|---|---|---|---|---|---|
| Cardioprotection | NaHS | Murine infarction model | Infarcted size and mortality significantly decreased | Upregulation of Bcl–2, demoted expression of Bax, IL–1β and Caspase 3 | |
| JKs | H9C2 cardiomyoblasts & murine ischemia/reperfusion model | A dose–dependent | |||
| inhibition in cell viability; significantly reduced AAR/LV and INS/AAR | |||||
| S–diclofenac | Rabbit model | Improved reperfusion pressure, anti–ischemic activity, activation of KATP channel | |||
| DAT–MSN | Cardiomyocyte, murine infarction model | inhibited myocardial inflammation, greater reduction in the infarct | Same as above | ||
| area and preserved cardiac ejection fraction | |||||
| GYY4137 | Cardiomyocyte, murine infarction | Infarcted size reduced, improved cardiac functions | Same as above | ||
| ADT–OH/PAM–PADT micelles | Rat cardiomyocytes | Rescue cells from apoptosis | |||
| PHDCs/SATO | H9C2 cardiomyoblasts | Mitigated Dox–induced toxicity | |||
| ALG–CHO/APTC/ADSC | ADSC/rat model | Improved heart function | Suppressed TNF–α, upregulation of genes related to angiogenesis and cardiac function | ||
| PFHy–MBs/CST | hCPCs | Improved cell growth | |||
| Atherosclerosis | NaHS | Apolipoprotein–E K.O. mice model & HUVEC | Antiatherogenic effect with promoted cell viability | Inhibited ICAM–1 and TNF–α signalling | |
| APA/SATO | HUVEC | Improved cell proliferation and migration | |||
| Chitosan/HA hydrogel/ACS14 | Platelet, rat model | Reduced inflammatory and AS lesion | |||
| Pulmonary arterial hypertension | LPM/ACS14 | PAH rat model, HPAEC | Delayed and reversed progression of PAH | Suppressed NF–κB–Snail pathway | |
| Wound healing | NaHS | HaCaT cell model, human epidermal melanocytes, HUVEC diabetic mice model | Promoted viability and differentiation | Promoted proliferation and differentiation via ATG5, TRP–1 signalling, angiogenesis via ANG–1, anti–inflammatory effect suppressing IL–6, TNF–α and MMP–9 | |
| Na2S | HUVEC, diabetic mice model | Suppressed inflammation, promoted migration and proliferation | Upregulation of KATP/P38/ERK/MAPK/VEGF signalling, and VEGFR2 transcription | ||
| NSHD1/PCL fibre | NIH 3T3, H9C2 cell model | Significantly prolonged release time, decreased ROS production | |||
| JK1/PCL fibre | NIH 3T3, mice model | Enhanced wound regeneration, prolonged release time | |||
| JK1/HA hydrogel | Mice model | Fast wound healing with enhanced cell proliferation and angiogenesis | Macrophage polarization towards M2 phenotype, suppressed TNF–α | ||
| JK1/SA hydrogel | L929 cell, rat model | Enhanced wound healing, promoted release profile | |||
| H2S/SA hydrogel | L929 cell, rat model | Promoted wound healing in a dose dependent manner | |||
| NaHS/rMaSp fibre | NIH 3T3, mice model | Promoted wound healing with EPC | |||
| SATO/PCL fibre | NHEK cells, diabetic mice model | Bacterial inhibition, promoted diabetic wound healing | |||
| Anti–bacterial | SATO/APA biofilm/dipeptides | Staphylococcus aureus | Inhibited bacterial growth | ||
| Intervertebral disc degeneration | JK1/Col hydrogel | Rat model, NP cell | Inhibited inflammatory process and cell apoptosis | Suppressing TNF–α, NF–κB, IL–1β expression and deactivation of P65 signalling | |
| Tissue engineering | GaOS/PLA membrane | Cardiac mesenchymal stem cell | Promoted proliferation with reduced oxidative damage | ||
| GYY4137/fibroin scaffold | Mouse fibroblast, hBMSC | Enhanced cell viability | |||
| Anti–cancer | Trisulfide/PEG–cholesteryl | MCF7 breast cancer cell | Suppressed tumourigenesis | Normalization of COL–1 expression | |
| ADT/AML | HepG2 cell, mice xenograft model | Reduction of tumour size, facilitate magnetic resonance imaging |
Table 1. Summary of applications of H2S donors and H2S–releasing biomaterials
| Application | H2S donor/biomaterials | Research model | Effects/outcome | Proposed mechanism | Reference |
|---|---|---|---|---|---|
| Cardioprotection | NaHS | Murine infarction model | Infarcted size and mortality significantly decreased | Upregulation of Bcl–2, demoted expression of Bax, IL–1β and Caspase 3 | |
| JKs | H9C2 cardiomyoblasts & murine ischemia/reperfusion model | A dose–dependent | |||
| inhibition in cell viability; significantly reduced AAR/LV and INS/AAR | |||||
| S–diclofenac | Rabbit model | Improved reperfusion pressure, anti–ischemic activity, activation of KATP channel | |||
| DAT–MSN | Cardiomyocyte, murine infarction model | inhibited myocardial inflammation, greater reduction in the infarct | Same as above | ||
| area and preserved cardiac ejection fraction | |||||
| GYY4137 | Cardiomyocyte, murine infarction | Infarcted size reduced, improved cardiac functions | Same as above | ||
| ADT–OH/PAM–PADT micelles | Rat cardiomyocytes | Rescue cells from apoptosis | |||
| PHDCs/SATO | H9C2 cardiomyoblasts | Mitigated Dox–induced toxicity | |||
| ALG–CHO/APTC/ADSC | ADSC/rat model | Improved heart function | Suppressed TNF–α, upregulation of genes related to angiogenesis and cardiac function | ||
| PFHy–MBs/CST | hCPCs | Improved cell growth | |||
| Atherosclerosis | NaHS | Apolipoprotein–E K.O. mice model & HUVEC | Antiatherogenic effect with promoted cell viability | Inhibited ICAM–1 and TNF–α signalling | |
| APA/SATO | HUVEC | Improved cell proliferation and migration | |||
| Chitosan/HA hydrogel/ACS14 | Platelet, rat model | Reduced inflammatory and AS lesion | |||
| Pulmonary arterial hypertension | LPM/ACS14 | PAH rat model, HPAEC | Delayed and reversed progression of PAH | Suppressed NF–κB–Snail pathway | |
| Wound healing | NaHS | HaCaT cell model, human epidermal melanocytes, HUVEC diabetic mice model | Promoted viability and differentiation | Promoted proliferation and differentiation via ATG5, TRP–1 signalling, angiogenesis via ANG–1, anti–inflammatory effect suppressing IL–6, TNF–α and MMP–9 | |
| Na2S | HUVEC, diabetic mice model | Suppressed inflammation, promoted migration and proliferation | Upregulation of KATP/P38/ERK/MAPK/VEGF signalling, and VEGFR2 transcription | ||
| NSHD1/PCL fibre | NIH 3T3, H9C2 cell model | Significantly prolonged release time, decreased ROS production | |||
| JK1/PCL fibre | NIH 3T3, mice model | Enhanced wound regeneration, prolonged release time | |||
| JK1/HA hydrogel | Mice model | Fast wound healing with enhanced cell proliferation and angiogenesis | Macrophage polarization towards M2 phenotype, suppressed TNF–α | ||
| JK1/SA hydrogel | L929 cell, rat model | Enhanced wound healing, promoted release profile | |||
| H2S/SA hydrogel | L929 cell, rat model | Promoted wound healing in a dose dependent manner | |||
| NaHS/rMaSp fibre | NIH 3T3, mice model | Promoted wound healing with EPC | |||
| SATO/PCL fibre | NHEK cells, diabetic mice model | Bacterial inhibition, promoted diabetic wound healing | |||
| Anti–bacterial | SATO/APA biofilm/dipeptides | Staphylococcus aureus | Inhibited bacterial growth | ||
| Intervertebral disc degeneration | JK1/Col hydrogel | Rat model, NP cell | Inhibited inflammatory process and cell apoptosis | Suppressing TNF–α, NF–κB, IL–1β expression and deactivation of P65 signalling | |
| Tissue engineering | GaOS/PLA membrane | Cardiac mesenchymal stem cell | Promoted proliferation with reduced oxidative damage | ||
| GYY4137/fibroin scaffold | Mouse fibroblast, hBMSC | Enhanced cell viability | |||
| Anti–cancer | Trisulfide/PEG–cholesteryl | MCF7 breast cancer cell | Suppressed tumourigenesis | Normalization of COL–1 expression | |
| ADT/AML | HepG2 cell, mice xenograft model | Reduction of tumour size, facilitate magnetic resonance imaging |
| 1. |
Olas, B. Hydrogen sulfide in signaling pathways. Clin Chim Acta. 2015, 439, 212-218.
doi: 10.1016/j.cca.2014.10.037 URL |
| 2. |
Martelli, A.; Testai, L.; Marino, A.; Breschi, M. C.; Da Settimo, F.; Calderone, V. Hydrogen sulphide: biopharmacological roles in the cardiovascular system and pharmaceutical perspectives. Curr Med Chem. 2012, 19, 3325-3336.
doi: 10.2174/092986712801215928 URL |
| 3. |
Li, L.; Hsu, A.; Moore, P. K. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation--a tale of three gases! Pharmacol Ther. 2009, 123, 386-400.
doi: 10.1016/j.pharmthera.2009.05.005 URL |
| 4. |
Wang, R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792-1798.
doi: 10.1096/fj.02-0211hyp URL |
| 5. |
Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996, 16, 1066-1071.
doi: 10.1523/JNEUROSCI.16-03-01066.1996 URL |
| 6. |
Peleli, M.; Zampas, P.; Papapetropoulos, A. Hydrogen sulfide and the kidney: physiological roles, contribution to pathophysiology, and therapeutic potential. Antioxid Redox Signal. 2022, 36, 220-243.
doi: 10.1089/ars.2021.0014 URL |
| 7. |
Mani, S.; Untereiner, A.; Wu, L.; Wang, R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid Redox Signal. 2014, 20, 805-817.
doi: 10.1089/ars.2013.5324 URL |
| 8. |
Feliers, D.; Lee, H. J.; Kasinath, B. S. Hydrogen sulfide in renal physiology and disease. Antioxid Redox Signal. 2016, 25, 720-731.
doi: 10.1089/ars.2015.6596 URL |
| 9. |
Hu, L. F.; Lu, M.; Hon Wong, P. T.; Bian, J. S. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011, 15, 405-419.
doi: 10.1089/ars.2010.3517 URL |
| 10. |
Sen, U.; Pushpakumar, S. B.; Amin, M. A.; Tyagi, S. C. Homocysteine in renovascular complications: hydrogen sulfide is a modulator and plausible anaerobic ATP generator. Nitric Oxide. 2014, 41, 27-37.
doi: 10.1016/j.niox.2014.06.006 URL |
| 11. |
Predmore, B. L.; Lefer, D. J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 2012, 17, 119-140.
doi: 10.1089/ars.2012.4612 URL |
| 12. |
Popov, D. An outlook on vascular hydrogen sulphide effects, signalling, and therapeutic potential. Arch Physiol Biochem. 2013, 119, 189-194.
doi: 10.3109/13813455.2013.803578 URL |
| 13. |
Kan, J.; Guo, W.; Huang, C.; Bao, G.; Zhu, Y.; Zhu, Y. Z. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid Redox Signal. 2014, 20, 2303-2316.
doi: 10.1089/ars.2013.5449 URL |
| 14. |
Qiu, X.; Villalta, J.; Lin, G.; Lue, T. F. Role of hydrogen sulfide in the physiology of penile erection. J Androl. 2012, 33, 529-535.
doi: 10.2164/jandrol.111.014936 URL |
| 15. |
Lefer, D. J. Potential importance of alterations in hydrogen sulphide (H2S) bioavailability in diabetes. Br J Pharmacol. 2008, 155, 617-619.
doi: 10.1038/bjp.2008.359 URL |
| 16. |
Gupta, S.; Kühnisch, J.; Mustafa, A.; Lhotak, S.; Schlachterman, A.; Slifker, M. J.; Klein-Szanto, A.; High, K. A.; Austin, R. C.; Kruger, W. D. Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 2009, 23, 883-893.
doi: 10.1096/fj.08-120584 URL |
| 17. |
Fiorucci, S.; Antonelli, E.; Distrutti, E.; Rizzo, G.; Mencarelli, A.; Orlandi, S.; Zanardo, R.; Renga, B.; Di Sante, M.; Morelli, A.; Cirino, G.; Wallace, J. L. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005, 129, 1210-1224.
doi: 10.1053/j.gastro.2005.07.060 URL |
| 18. |
Denizalti, M.; Bozkurt, T. E.; Akpulat, U.; Sahin-Erdemli, I.; Abacıoğlu, N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2011, 383, 509-517.
doi: 10.1007/s00210-011-0601-6 URL |
| 19. |
Brancaleone, V.; Roviezzo, F.; Vellecco, V.; De Gruttola, L.; Bucci, M.; Cirino, G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br J Pharmacol. 2008, 155, 673-680.
doi: 10.1038/bjp.2008.296 URL |
| 20. |
Calvert, J. W.; Coetzee, W. A.; Lefer, D. J. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010, 12, 1203-1217.
doi: 10.1089/ars.2009.2882 URL |
| 21. |
Hartle, M. D.; Pluth, M. D. A practical guide to working with H(2)S at the interface of chemistry and biology. Chem Soc Rev. 2016, 45, 6108-6117.
doi: 10.1039/C6CS00212A URL |
| 22. |
Zhao, Y.; Wang, H.; Huang, H.; Xiao, Q.; Xu, Y.; Guo, Z.; Xie, H.; Shao, J.; Sun, Z.; Han, W.; Yu, X. F.; Li, P.; Chu, P. K. Surface coordination of black phosphorus for robust air and water stability. Angew Chem Int Ed Engl. 2016, 55, 5003-5007.
doi: 10.1002/anie.201512038 URL |
| 23. |
Wang, R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens. 2011, 20, 107-112.
doi: 10.1097/MNH.0b013e3283430651 URL |
| 24. | Chen, W. L.; Niu, Y. Y.; Jiang, W. Z.; Tang, H. L.; Zhang, C.; Xia, Q. M.; Tang, X. Q. Neuroprotective effects of hydrogen sulfide and the underlying signaling pathways. Rev Neurosci. 2015, 26, 129-142. |
| 25. |
Pieretti, J. C.; Junho, C. V. C.; Carneiro-Ramos, M. S.; Seabra, A. B. H(2)S- and NO-releasing gasotransmitter platform: A crosstalk signaling pathway in the treatment of acute kidney injury. Pharmacol Res. 2020, 161, 105121.
doi: 10.1016/j.phrs.2020.105121 URL |
| 26. |
Filipovic, M. R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H(2)S signaling through persulfidation. Chem Rev. 2018, 118, 1253-1337.
doi: 10.1021/acs.chemrev.7b00205 URL |
| 27. |
Reiffenstein, R. J.; Hulbert, W. C.; Roth, S. H. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992, 32, 109-134.
doi: 10.1146/annurev.pa.32.040192.000545 URL |
| 28. | Peng, B.; Xian, M. Hydrogen sulfide detection using nucleophilic substitution-cyclization-based fluorescent probes. Methods Enzymol. 2015, 554, 47-62. |
| 29. | Chang, H. W.; Frey, G.; Liu, H.; Xing, C.; Steinman, L.; Boyle, W. J.; Short, J. M. Generating tumor-selective conditionally active biologic anti-CTLA4 antibodies via protein-associated chemical switches. Proc Natl Acad Sci U S A. 2021, 118, e2020606118. |
| 30. | Baseggio Conrado, A.; Capuozzo, E.; Mosca, L.; Francioso, A.; Fontana, M. Thiotaurine: from chemical and biological properties to role in H(2)S signaling. Adv Exp Med Biol. 2019, 1155, 755-771. |
| 31. | Olson, K. R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta. 2009, 1787, 856-863. |
| 32. |
Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008-6016.
doi: 10.1093/emboj/20.21.6008 URL |
| 33. |
Savage, J. C.; Gould, D. H. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr. 1990, 526, 540-545.
doi: 10.1016/S0378-4347(00)82537-2 URL |
| 34. |
Goodwin, L. R.; Francom, D.; Dieken, F. P.; Taylor, J. D.; Warenycia, M. W.; Reiffenstein, R. J.; Dowling, G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol. 1989, 13, 105-109.
doi: 10.1093/jat/13.2.105 URL |
| 35. | Whitfield, N. L.; Kreimier, E. L.; Verdial, F. C.; Skovgaard, N.; Olson, K. R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol. 2008, 294, R 1930-1937. |
| 36. |
Levitt, M. D.; Abdel-Rehim, M. S.; Furne, J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal. 2011, 15, 373-378.
doi: 10.1089/ars.2010.3525 URL |
| 37. |
Warenycia, M. W.; Goodwin, L. R.; Benishin, C. G.; Reiffenstein, R. J.; Francom, D. M.; Taylor, J. D.; Dieken, F. P. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989, 38, 973-981.
doi: 10.1016/0006-2952(89)90288-8 URL |
| 38. |
Cao, X.; Ding, L.; Xie, Z. Z.; Yang, Y.; Whiteman, M.; Moore, P. K.; Bian, J. S. A review of hydrogen sulfide synthesis, metabolism, and measurement: is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid Redox Signal. 2019, 31, 1-38.
doi: 10.1089/ars.2017.7058 URL |
| 39. |
He, F.; Cui, X.; Ren, J. A Novel QCM-based biosensor for detection of microorganisms producing hydrogen sulfide. Anal Lett. 2008, 41, 2697-2709.
doi: 10.1080/00032710802238028 URL |
| 40. |
Oh, G. S.; Pae, H. O.; Lee, B. S.; Kim, B. N.; Kim, J. M.; Kim, H. R.; Jeon, S. B.; Jeon, W. K.; Chae, H. J.; Chung, H. T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006, 41, 106-119.
doi: 10.1016/j.freeradbiomed.2006.03.021 URL |
| 41. |
DeLeon, E. R.; Stoy, G. F.; Olson, K. R. Passive loss of hydrogen sulfide in biological experiments. Anal Biochem. 2012, 421, 203-207.
doi: 10.1016/j.ab.2011.10.016 URL |
| 42. |
Liu, Y. H.; Lu, M.; Hu, L. F.; Wong, P. T.; Webb, G. D.; Bian, J. S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal. 2012, 17, 141-185.
doi: 10.1089/ars.2011.4005 URL |
| 43. |
Levinn, C. M.; Cerda, M. M.; Pluth, M. D. Activatable small-molecule hydrogen sulfide donors. Antioxid Redox Signal. 2020, 32, 96-109.
doi: 10.1089/ars.2019.7841 URL |
| 44. |
Hao, Y.; Wang, H.; Fang, L.; Bian, J.; Gao, Y.; Li, C. H2S donor and bone metabolism. Front Pharmacol. 2021, 12, 661601.
doi: 10.3389/fphar.2021.661601 URL |
| 45. |
Kang, J.; Li, Z.; Organ, C. L.; Park, C. M.; Yang, C. T.; Pacheco, A.; Wang, D.; Lefer, D. J.; Xian, M. pH-controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J Am Chem Soc. 2016, 138, 6336-6339.
doi: 10.1021/jacs.6b01373 URL |
| 46. |
Zheng, Y.; Yu, B.; De La Cruz, L. K.; Roy Choudhury, M.; Anifowose, A.; Wang, B. Toward hydrogen sulfide based therapeutics: critical drug delivery and developability issues. Med Res Rev. 2018, 38, 57-100.
doi: 10.1002/med.21433 URL |
| 47. |
Zhang, N.; Hu, P.; Wang, Y.; Tang, Q.; Zheng, Q.; Wang, Z.; He, Y. A reactive oxygen species (ROS) activated hydrogen sulfide (H(2)S) donor with self-reporting fluorescence. ACS Sens. 2020, 5, 319-326.
doi: 10.1021/acssensors.9b01093 URL |
| 48. |
Forrester, S. J.; Kikuchi, D. S.; Hernandes, M. S.; Xu, Q.; Griendling, K. K. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018, 122, 877-902.
doi: 10.1161/CIRCRESAHA.117.311401 URL |
| 49. |
Zhao, Y.; Pluth, M. D. Hydrogen sulfide donors activated by reactive oxygen species. Angew Chem Int Ed Engl. 2016, 55, 14638-14642.
doi: 10.1002/anie.201608052 URL |
| 50. |
Powell, C. R.; Dillon, K. M.; Matson, J. B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem Pharmacol. 2018, 149, 110-123.
doi: 10.1016/j.bcp.2017.11.014 URL |
| 51. |
Zhao, Y.; Kang, J.; Park, C. M.; Bagdon, P. E.; Peng, B.; Xian, M. Thiol-activated gem-dithiols: a new class of controllable hydrogen sulfide donors. Org Lett. 2014, 16, 4536-4539.
doi: 10.1021/ol502088m URL |
| 52. |
Wu, J.; Li, Y.; He, C.; Kang, J.; Ye, J.; Xiao, Z.; Zhu, J.; Chen, A.; Feng, S.; Li, X.; Xiao, J.; Xian, M.; Wang, Q. Novel H(2)S releasing nanofibrous coating for in vivo dermal wound regeneration. ACS Appl Mater Interfaces. 2016, 8, 27474-27481.
doi: 10.1021/acsami.6b06466 URL |
| 53. |
Schneider, L. A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. 2007, 298, 413-420.
doi: 10.1007/s00403-006-0713-x URL |
| 54. |
Zhuang, R.; Guo, L.; Du, J.; Wang, S.; Li, J.; Liu, Y. Exogenous hydrogen sulfide inhibits oral mucosal wound-induced macrophage activation via the NF-κB pathway. Oral Dis. 2018, 24, 793-801.
doi: 10.1111/odi.12838 URL |
| 55. |
Goren, I.; Köhler, Y.; Aglan, A.; Pfeilschifter, J.; Beck, K. F.; Frank, S. Increase of cystathionine-γ-lyase (CSE) during late wound repair: Hydrogen sulfide triggers cytokeratin 10 expression in keratinocytes. Nitric Oxide. 2019, 87, 31-42.
doi: 10.1016/j.niox.2019.03.004 URL |
| 56. |
Wu, J.; Chen, A.; Zhou, Y.; Zheng, S.; Yang, Y.; An, Y.; Xu, K.; He, H.; Kang, J.; Luckanagul, J. A.; Xian, M.; Xiao, J.; Wang, Q. Novel H(2)S-Releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials. 2019, 222, 119398.
doi: 10.1016/j.biomaterials.2019.119398 URL |
| 57. |
Zhao, X.; Liu, L.; An, T.; Xian, M.; Luckanagul, J. A.; Su, Z.; Lin, Y.; Wang, Q. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020, 104, 85-94.
doi: 10.1016/j.actbio.2019.12.032 URL |
| 58. |
Xu, W.; Watanabe, K.; Mizukami, Y.; Yamamoto, Y.; Suzuki, T. Hydrogen sulfide suppresses the proliferation of intestinal epithelial cells through cell cycle arrest. Arch Biochem Biophys. 2021, 712, 109044.
doi: 10.1016/j.abb.2021.109044 URL |
| 59. |
Zhang, H.; Bai, Z.; Zhu, L.; Liang, Y.; Fan, X.; Li, J.; Wen, H.; Shi, T.; Zhao, Q.; Wang, Z. Hydrogen sulfide donors: Therapeutic potential in anti-atherosclerosis. Eur J Med Chem. 2020, 205, 112665.
doi: 10.1016/j.ejmech.2020.112665 URL |
| 60. |
Jewell, C.; Bennett, P.; Mutch, E.; Ackermann, C.; Williams, F. M. Inter-individual variability in esterases in human liver. Biochem Pharmacol. 2007, 74, 932-939.
doi: 10.1016/j.bcp.2007.06.022 URL |
| 61. |
Bełtowski, J. Hydrogen sulfide in pharmacology and medicine--an update. Pharmacol Rep. 2015, 67, 647-658.
doi: 10.1016/j.pharep.2015.01.005 URL |
| 62. |
Zheng, Y.; Ji, X.; Ji, K.; Wang, B. Hydrogen sulfide prodrugs-a review. Acta Pharm Sin B. 2015, 5, 367-377.
doi: 10.1016/j.apsb.2015.06.004 URL |
| 63. | Wen, Y. D.; Wang, H.; Zhu, Y. Z. The Drug Developments of hydrogen sulfide on cardiovascular disease. Oxid Med Cell Longev. 2018, 2018, 4010395. |
| 64. |
Zhao, Y.; Biggs, T. D.; Xian, M. Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem Commun (Camb). 2014, 50, 11788-11805.
doi: 10.1039/C4CC00968A URL |
| 65. |
Feng, S.; Zhao, Y.; Xian, M.; Wang, Q. Biological thiols-triggered hydrogen sulfide releasing microfibers for tissue engineering applications. Acta Biomater. 2015, 27, 205-213.
doi: 10.1016/j.actbio.2015.09.010 URL |
| 66. |
Chen, M.; Li, Y. F.; Besenbacher, F. Electrospun nanofibers-mediated on-demand drug release. Adv Healthc Mater. 2014, 3, 1721-1732.
doi: 10.1002/adhm.201400166 URL |
| 67. |
Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv Healthc Mater. 2012, 1, 10-25.
doi: 10.1002/adhm.201100021 URL |
| 68. | Venugopal, J.; Low, S.; Choon, A. T.; Ramakrishna, S. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater. 2008, 84, 34-48. |
| 69. |
Rowley, J. A.; Madlambayan, G.; Mooney, D. J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999, 20, 45-53.
doi: 10.1016/S0142-9612(98)00107-0 URL |
| 70. |
Wu, A. T.; Aoki, T.; Sakoda, M.; Ohta, S.; Ichimura, S.; Ito, T.; Ushida, T.; Furukawa, K. S. Enhancing osteogenic differentiation of MC3T3-E1 cells by immobilizing inorganic polyphosphate onto hyaluronic acid hydrogel. Biomacromolecules. 2015, 16, 166-173.
doi: 10.1021/bm501356c URL |
| 71. |
Lee, K. Y.; Mooney, D. J. Alginate: properties and biomedical applications. Prog Polym Sci. 2012, 37, 106-126.
doi: 10.1016/j.progpolymsci.2011.06.003 URL |
| 72. |
Foster, J. C.; Radzinski, S. C.; Zou, X.; Finkielstein, C. V.; Matson, J. B. H(2)S-releasing polymer micelles for studying selective cell toxicity. Mol Pharm. 2017, 14, 1300-1306.
doi: 10.1021/acs.molpharmaceut.6b01117 URL |
| 73. |
Carrazzone, R. J.; Foster, J. C.; Li, Z.; Matson, J. B. Tuning small molecule release from polymer micelles: Varying H(2)S release through cross linking in the micelle core. Eur Polym J. 2020, 141, 110077.
doi: 10.1016/j.eurpolymj.2020.110077 URL |
| 74. |
Wang, Y.; Kaur, K.; Scannelli, S. J.; Bitton, R.; Matson, J. B. Self-assembled nanostructures regulate H(2)S release from constitutionally isomeric peptides. J Am Chem Soc. 2018, 140, 14945-14951.
doi: 10.1021/jacs.8b09320 URL |
| 75. |
Kaur, K.; Wang, Y.; Matson, J. B. Linker-regulated H(2)S release from aromatic peptide amphiphile hydrogels. Biomacromolecules. 2020, 21, 1171-1178.
doi: 10.1021/acs.biomac.9b01600 URL |
| 76. |
Longchamp, A.; Kaur, K.; Macabrey, D.; Dubuis, C.; Corpataux, J. M.; Déglise, S.; Matson, J. B.; Allagnat, F. Hydrogen sulfide-releasing peptide hydrogel limits the development of intimal hyperplasia in human vein segments. Acta Biomater. 2019, 97, 374-384.
doi: 10.1016/j.actbio.2019.07.042 URL |
| 77. |
Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007, 6, 917-935.
doi: 10.1038/nrd2425 URL |
| 78. |
Szabó, G.; Veres, G.; Radovits, T.; Gero, D.; Módis, K.; Miesel-Gröschel, C.; Horkay, F.; Karck, M.; Szabó, C. Cardioprotective effects of hydrogen sulfide. Nitric Oxide. 2011, 25, 201-210.
doi: 10.1016/j.niox.2010.11.001 URL |
| 79. |
Pan, L. L.; Liu, X. H.; Gong, Q. H.; Yang, H. B.; Zhu, Y. Z. Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxid Redox Signal. 2012, 17, 106-118.
doi: 10.1089/ars.2011.4349 URL |
| 80. |
Zhu, Y. Z.; Wang, Z. J.; Ho, P.; Loke, Y. Y.; Zhu, Y. C.; Huang, S. H.; Tan, C. S.; Whiteman, M.; Lu, J.; Moore, P. K. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol (1985). 2007, 102, 261-268.
doi: 10.1152/japplphysiol.00096.2006 URL |
| 81. |
Wang, X.; Wang, Q.; Guo, W.; Zhu, Y. Z. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: a mechanism through cardiac mitochondrial protection. Biosci Rep. 2011, 31, 87-98.
doi: 10.1042/BSR20100003 URL |
| 82. |
Zhuo, Y.; Chen, P. F.; Zhang, A. Z.; Zhong, H.; Chen, C. Q.; Zhu, Y. Z. Cardioprotective effect of hydrogen sulfide in ischemic reperfusion experimental rats and its influence on expression of survivin gene. Biol Pharm Bull. 2009, 32, 1406-1410.
doi: 10.1248/bpb.32.1406 URL |
| 83. |
Rossoni, G.; Sparatore, A.; Tazzari, V.; Manfredi, B.; Del Soldato, P.; Berti, F. The hydrogen sulphide-releasing derivative of diclofenac protects against ischaemia-reperfusion injury in the isolated rabbit heart. Br J Pharmacol. 2008, 153, 100-109.
doi: 10.1038/sj.bjp.0707540 URL |
| 84. |
Sun, X.; Wang, W.; Dai, J.; Jin, S.; Huang, J.; Guo, C.; Wang, C.; Pang, L.; Wang, Y. A long-term and slow-releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Sci Rep. 2017, 7, 3541.
doi: 10.1038/s41598-017-03941-0 URL |
| 85. |
Takatani-Nakase, T.; Katayama, M.; Matsui, C.; Hanaoka, K.; van der Vlies, A. J.; Takahashi, K.; Nakase, I.; Hasegawa, U. Hydrogen sulfide donor micelles protect cardiomyocytes from ischemic cell death. Mol Biosyst. 2017, 13, 1705-1708.
doi: 10.1039/C7MB00191F URL |
| 86. |
Wang, Y.; Zhao, X.; Jin, H.; Wei, H.; Li, W.; Bu, D.; Tang, X.; Ren, Y.; Tang, C.; Du, J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009, 29, 173-179.
doi: 10.1161/ATVBAHA.108.179333 URL |
| 87. |
Li, W.; Tang, C.; Jin, H.; Du, J. Regulatory effects of sulfur dioxide on the development of atherosclerotic lesions and vascular hydrogen sulfide in atherosclerotic rats. Atherosclerosis. 2011, 215, 323-330.
doi: 10.1016/j.atherosclerosis.2010.12.037 URL |
| 88. |
Li, L.; Whiteman, M.; Moore, P. K. Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock. J Cell Mol Med. 2009, 13, 2684-2692.
doi: 10.1111/j.1582-4934.2008.00610.x URL |
| 89. | Pan, L. L.; Liu, X. H.; Gong, Q. H.; Wu, D.; Zhu, Y. Z. Hydrogen sulfide attenuated tumor necrosis factor-α-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS One. 2011, 6, e19766. |
| 90. |
Zhang, H.; Hao, L. Z.; Pan, J. A.; Gao, Q.; Zhang, J. F.; Kankala, R. K.; Wang, S. B.; Chen, A. Z.; Zhang, H. L. Microfluidic fabrication of inhalable large porous microspheres loaded with H(2)S-releasing aspirin derivative for pulmonary arterial hypertension therapy. J Control Release. 2021, 329, 286-298.
doi: 10.1016/j.jconrel.2020.11.060 URL |
| 91. |
Wang, Y.; Matson, J. B. Supramolecular nanostructures with tunable donor loading for controlled H(2)S release. ACS Appl Bio Mater. 2019, 2, 5093-5098.
doi: 10.1021/acsabm.9b00768 URL |
| 92. | Lu, B.; Han, X.; Zhao, A.; Luo, D.; Maitz, M. F.; Wang, H.; Yang, P.; Huang, N. Intelligent H2S release coating for regulating vascular remodeling. Bioact Mater. 2021, 6, 1040-1050. |
| 93. |
Liang, W.; Chen, J.; Li, L.; Li, M.; Wei, X.; Tan, B.; Shang, Y.; Fan, G.; Wang, W.; Liu, W. Conductive hydrogen sulfide-releasing hydrogel encapsulating ADSCs for myocardial infarction treatment. ACS Appl Mater Interfaces. 2019, 11, 14619-14629.
doi: 10.1021/acsami.9b01886 URL |
| 94. |
Mauretti, A.; Neri, A.; Kossover, O.; Seliktar, D.; Nardo, P. D.; Melino, S. Design of a novel composite H2S-releasing hydrogel for cardiac tissue repair. Macromol Biosci. 2016, 16, 847-858.
doi: 10.1002/mabi.201500430 URL |
| 95. | Xiao, Q.; Xiong, L.; Tang, J.; Li, L.; Li, L. Hydrogen Sulfide in Skin Diseases: A Novel Mediator and Therapeutic Target. Oxid Med Cell Longev. 2021, 2021, 6652086. |
| 96. | Oyoshi, M. K.; He, R.; Kumar, L.; Yoon, J.; Geha, R. S. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol. 2009, 102, 135-226. |
| 97. |
Angel, P.; Szabowski, A. Function of AP-1 target genes in mesenchymal-epithelial cross-talk in skin. Biochem Pharmacol. 2002, 64, 949-956.
doi: 10.1016/S0006-2952(02)01158-9 URL |
| 98. |
Lerman, O. Z.; Galiano, R. D.; Armour, M.; Levine, J. P.; Gurtner, G. C. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003, 162, 303-312.
doi: 10.1016/S0002-9440(10)63821-7 URL |
| 99. | Xuan, Y. H.; Huang, B. B.; Tian, H. S.; Chi, L. S.; Duan, Y. M.; Wang, X.; Zhu, Z. X.; Cai, W. H.; Zhu, Y. T.; Wei, T. M.; Ye, H. B.; Cong, W. T.; Jin, L. T. High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS One. 2014, 9, e108182. |
| 100. |
Hu, S. C.; Lan, C. E. High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. J Dermatol Sci. 2016, 84, 121-127.
doi: 10.1016/j.jdermsci.2016.07.008 URL |
| 101. |
Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014, 31, 817-836.
doi: 10.1007/s12325-014-0140-x URL |
| 102. |
Xie, X.; Dai, H.; Zhuang, B.; Chai, L.; Xie, Y.; Li, Y. Exogenous hydrogen sulfide promotes cell proliferation and differentiation by modulating autophagy in human keratinocytes. Biochem Biophys Res Commun. 2016, 472, 437-443.
doi: 10.1016/j.bbrc.2016.01.047 URL |
| 103. |
Ying, J.; Wang, Q.; Jiang, M.; Wang, X.; Liu, W.; Wang, X.; Zhang, C.; Xiang, L. Hydrogen sulfide promotes cell proliferation and melanin synthesis in primary human epidermal melanocytes. Skin Pharmacol Physiol. 2020, 33, 61-68.
doi: 10.1159/000506818 URL |
| 104. |
Liu, F.; Chen, D. D.; Sun, X.; Xie, H. H.; Yuan, H.; Jia, W.; Chen, A. F. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014, 63, 1763-1778.
doi: 10.2337/db13-0483 URL |
| 105. |
Zhao, H.; Lu, S.; Chai, J.; Zhang, Y.; Ma, X.; Chen, J.; Guan, Q.; Wan, M.; Liu, Y. Hydrogen sulfide improves diabetic wound healing in ob/ob mice via attenuating inflammation. J Diabetes Complications. 2017, 31, 1363-1369.
doi: 10.1016/j.jdiacomp.2017.06.011 URL |
| 106. |
Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M. G.; Branski, L. K.; Herndon, D. N.; Wang, R.; Szabó, C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009, 106, 21972-21977.
doi: 10.1073/pnas.0908047106 URL |
| 107. |
Yang, C. T.; Chen, L.; Chen, W. L.; Li, N.; Chen, M. J.; Li, X.; Zheng, X.; Zhao, Y. Z.; Wu, Y. X.; Xian, M.; Liu, J. Hydrogen sulfide primes diabetic wound to close through inhibition of NETosis. Mol Cell Endocrinol. 2019, 480, 74-82.
doi: 10.1016/j.mce.2018.10.013 URL |
| 108. |
Saha, S.; Chakraborty, P. K.; Xiong, X.; Dwivedi, S. K.; Mustafi, S. B.; Leigh, N. R.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathionine β-synthase regulates endothelial function via protein S-sulfhydration. FASEB J. 2016, 30, 441-456.
doi: 10.1096/fj.15-278648 URL |
| 109. |
Whiteman, M.; Gooding, K. M.; Whatmore, J. L.; Ball, C. I.; Mawson, D.; Skinner, K.; Tooke, J. E.; Shore, A. C. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010, 53, 1722-1726.
doi: 10.1007/s00125-010-1761-5 URL |
| 110. |
Jain, S. K.; Bull, R.; Rains, J. L.; Bass, P. F.; Levine, S. N.; Reddy, S.; McVie, R.; Bocchini, J. A. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal. 2010, 12, 1333-1337.
doi: 10.1089/ars.2009.2956 URL |
| 111. |
Szabó, C.; Papapetropoulos, A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol. 2011, 164, 853-865.
doi: 10.1111/j.1476-5381.2010.01191.x URL |
| 112. |
Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M. R. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. Int J Biol Macromol. 2019, 140, 1260-1268.
doi: 10.1016/j.ijbiomac.2019.08.207 URL |
| 113. | Kannon, G. A.; Garrett, A. B. Moist wound healing with occlusive dressings. A clinical review. Dermatol Surg. 1995, 21, 583-590. |
| 114. |
Lin, S. Y.; Chen, K. S.; Run-Chu, L. Design and evaluation of drug-loaded wound dressing having thermoresponsive, adhesive, absorptive and easy peeling properties. Biomaterials. 2001, 22, 2999-3004.
doi: 10.1016/S0142-9612(01)00046-1 URL |
| 115. |
Nazarnezhada, S.; Abbaszadeh-Goudarzi, G.; Samadian, H.; Khaksari, M.; Ghatar, J. M.; Khastar, H.; Rezaei, N.; Mousavi, S. R.; Shirian, S.; Salehi, M. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: In vitro and in vivo study. Int J Biol Macromol. 2020, 164, 3323-3331.
doi: 10.1016/j.ijbiomac.2020.08.233 URL |
| 116. |
Lian, J.; Ju, G.; Cai, X.; Cai, Y.; Li, C.; Ma, S.; Cao, Y. Nanofibrous membrane dressings loaded with sodium hydrogen sulfide/endothelial progenitor cells promote wound healing. Front Bioeng Biotechnol. 2021, 9, 657549.
doi: 10.3389/fbioe.2021.657549 URL |
| 117. |
Liu, D.; Liao, Y.; Cornel, E. J.; Lv, M.; Wu, T.; Zhang, X.; Fan, L.; Sun, M.; Zhu, Y.; Fan, Z.; Du, J. Polymersome wound dressing spray capable of bacterial inhibition and H2S generation for complete diabetic wound healing. Chem Mater. 2021, 33, 7972-7985.
doi: 10.1021/acs.chemmater.1c01872 URL |
| 118. |
Qian, Y.; Altamimi, A.; Yates, S. A.; Sarkar, S.; Cochran, M.; Zhou, M.; Levi-Polyachenko, N.; Matson, J. B. H(2)S-releasing amphiphilic dipeptide hydrogels are potent S. aureus biofilm disruptors. Biomater Sci. 2020, 8, 2564-2576.
doi: 10.1039/D0BM00241K URL |
| 119. |
Zheng, Z.; Chen, A.; He, H.; Chen, Y.; Chen, J.; Albashari, A. A.; Li, J.; Yin, J.; He, Z.; Wang, Q.; Wu, J.; Wang, Q.; Kang, J.; Xian, M.; Wang, X.; Xiao, J. pH and enzyme dual-responsive release of hydrogen sulfide for disc degeneration therapy. J Mater Chem B. 2019, 7, 611-618.
doi: 10.1039/C8TB02566E URL |
| 120. |
Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen sulfide-releasing fibrous membranes: potential patches for stimulating human stem cells proliferation and viability under oxidative stress. Int J Mol Sci. 2018, 19, 2368.
doi: 10.3390/ijms19082368 URL |
| 121. |
Raggio, R.; Bonani, W.; Callone, E.; Dirè, S.; Gambari, L.; Grassi, F.; Motta, A. Silk fibroin porous scaffolds loaded with a slow-releasing hydrogen sulfide agent (GYY4137) for applications of tissue engineering. ACS Biomater Sci Eng. 2018, 4, 2956-2966.
doi: 10.1021/acsbiomaterials.8b00212 URL |
| 122. |
Kim, B. Y.; Han, M. J.; Chung, A. S. Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells. Free Radic Biol Med. 2001, 30, 686-698.
doi: 10.1016/S0891-5849(00)00514-1 URL |
| 123. |
Murrell, G. A.; Francis, M. J.; Bromley, L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990, 265, 659-665.
doi: 10.1042/bj2650659 URL |
| 124. |
Toullec, A.; Gerald, D.; Despouy, G.; Bourachot, B.; Cardon, M.; Lefort, S.; Richardson, M.; Rigaill, G.; Parrini, M. C.; Lucchesi, C.; Bellanger, D.; Stern, M. H.; Dubois, T.; Sastre-Garau, X.; Delattre, O.; Vincent-Salomon, A.; Mechta-Grigoriou, F. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010, 2, 211-230.
doi: 10.1002/emmm.201000073 URL |
| 125. |
Dao, N. V.; Ercole, F.; Urquhart, M. C.; Kaminskas, L. M.; Nowell, C. J.; Davis, T. P.; Sloan, E. K.; Whittaker, M. R.; Quinn, J. F. Trisulfide linked cholesteryl PEG conjugate attenuates intracellular ROS and collagen-1 production in a breast cancer co-culture model. Biomater Sci. 2021, 9, 835-846.
doi: 10.1039/D0BM01544J URL |
| 126. |
Liu, Y.; Yang, F.; Yuan, C.; Li, M.; Wang, T.; Chen, B.; Jin, J.; Zhao, P.; Tong, J.; Luo, S.; Gu, N. Magnetic nanoliposomes as in situ microbubble bombers for multimodality image-guided cancer theranostics. ACS Nano. 2017, 11, 1509-1519.
doi: 10.1021/acsnano.6b06815 URL |
| [1] | Yiqiang Hu, Yuan Xiong, Ranyang Tao, Hang Xue, Lang Chen, Ze Lin, Adriana C. Panayi, Bobin Mi, Guohui Liu. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing [J]. Biomaterials Translational, 2022, 3(3): 188-200. |
| [2] | Shuqin Cao, Quan Yuan. An update of nanotopographical surfaces in modulating stem cell fate: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 55-64. |
| [3] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [4] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [5] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [6] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [7] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [8] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [9] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||