Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (2): 104-114.doi: 10.12336/biomatertransl.2023.02.005
• RESEARCH ARTICLE • Previous Articles Next Articles
Ross H. McWilliam )
)
Received:2023-01-27
Revised:2023-04-19
Accepted:2023-06-20
Online:2023-06-28
Published:2023-06-28
Contact:
Wenmiao Shu
E-mail:will.shu@strath.ac.uk
About author:Wenmiao Shu, will.shu@strath.ac.uk.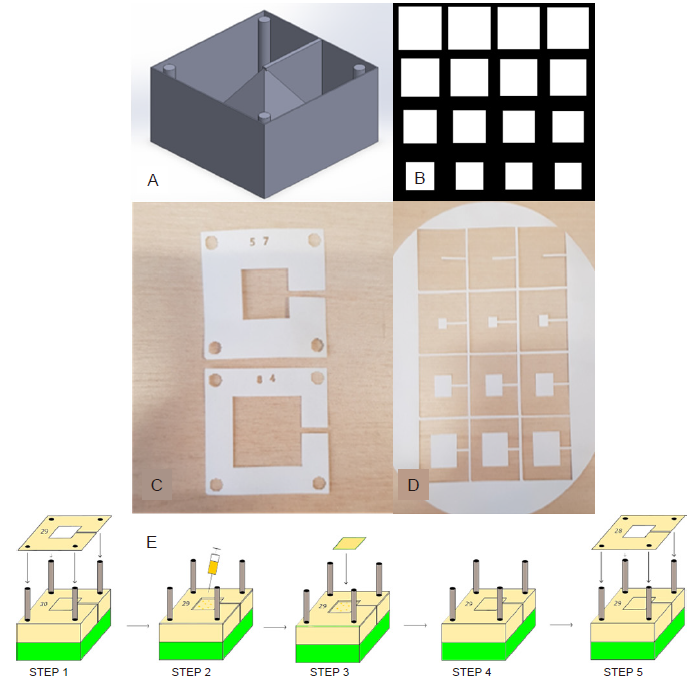
Figure 1. Design and assembly process for fabricating a 3D construct. (A) CAD model with 3D construction and surrounding box and cylinders. (B) Section of the array in InkScape showing different 2D slice shapes used to construct the pyramid. (C) Laser cut alignment slices with an appropriate number designating order. (D) Shape slices on the bulk material to be cross-referenced with alignment slice and placed accordingly on the construct. (E) Schematic of the steps involved in the assembly process, where accurate 3D reconstructions can be created from the 2D slices: Step 1 - placement of alignment slice 29 on the rig using the four holes; Step 2 - Deposition of a couple of drops of alginate solution using a syringe; Steps 3 and 4 - placement of the appropriate shape slice corresponding with alignment slice 29; Step 5 - starting the process again with alignment slice 28. This process continues until all slices are placed. 2D: two-dimensional; 3D: three-dimensional; CAD: computer-aided design. Created with Inventor? Vension 2018.
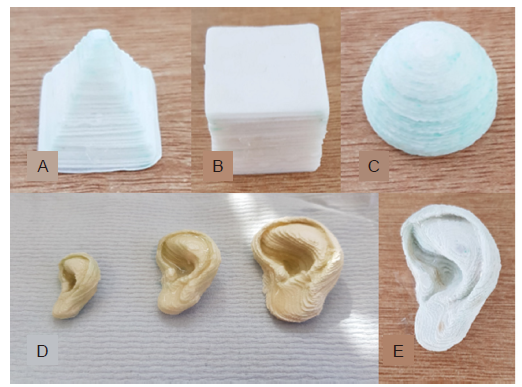
Figure 2. Examples of 3D demonstrators assembled according to the novel fabrication method. (A) Camera image of a pyramid. (B) Camera image of a successfully printed cube. (C) Camera image of a successfully printed hemisphere. (D) Three scale models of a human ear (200 μm slices) of three different sizes. (E) The corresponding ear with greater resolution (100 μm slices), which has excellent fidelity for anatomical size and accurately replicates the complex features of the human ear. Scale bars: 10 mm (A–C, E), 50 mm (D).
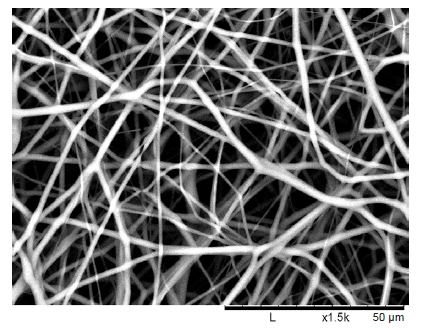
Figure 3. Desktop scanning electron microscope image of an electrospun polycaprolactone nanofibre mesh to be micromachined and used in in vitro testing. An average nanofibre diameter was 1.801 μm (n = 10) was found. Scale bars: 50 μm.
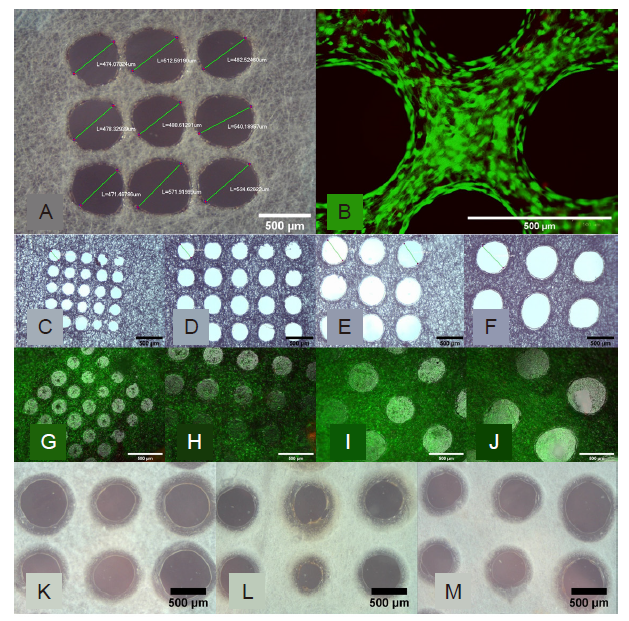
Figure 4. Morphological assessment of the perforated micromachined fibre mesh. (A, B) First cytotoxicity test of the 500 μm micromachined PLGA mesh. (A) Light Microscopy image of the micromachined array showing good circularity. (B) Live/dead image showing excellent cell viability and the holes clearly defined. (C–F) Light microscopy images of the 200 (C), 300 (D), 400 (E), and 500 (F) μm hole arrays, which show good circularity and even distribution. (G–J) Epifluorescent microscope images of live ADSCs on the micromachined fibre mesh with 200 (G), 300 (H), 400 (I), and 500 (J) μm hole arrays, with excellent cell viability seen across all samples, and ‘bridging' of cells across. (K–M) Light microscope images of three selected micromachined electrospun PCL fibre mesh samples, all micromachined with the same setting but with minor variances as a result of the non-uniform thickness of the PCL nanofibre mesh. Scale bars: 500 μm. PCL: polycaprolactone; PLGA: polylactic-co-glycolic acid.
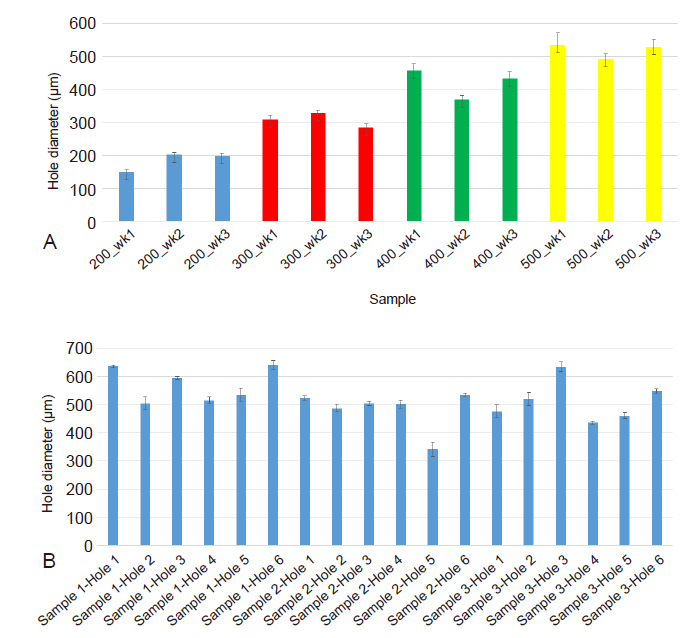
Figure 5. The average diameter of the micromachined perforations in the electrospun fibre mesh samples. (A) The actual average hole diameter of each array (n = 3 holes) compared to the intended hole diameter of the PLGA samples, where the average diameter can be seen to be close to the intended diameter and a clear difference between each sample type seen. (B) The average diameter of each hole (n = 3 holes) in three representative micromachined electrospun PCL fibre mesh samples, with a wider variance of values around the intended 500 μm hole diameter due to using the same laser settings on the non-uniform thickness of the PCL nanofibre mesh. 3D: three-dimensional; PCL: polycaprolactone; PLGA: polylactic-co-glycolic acid.
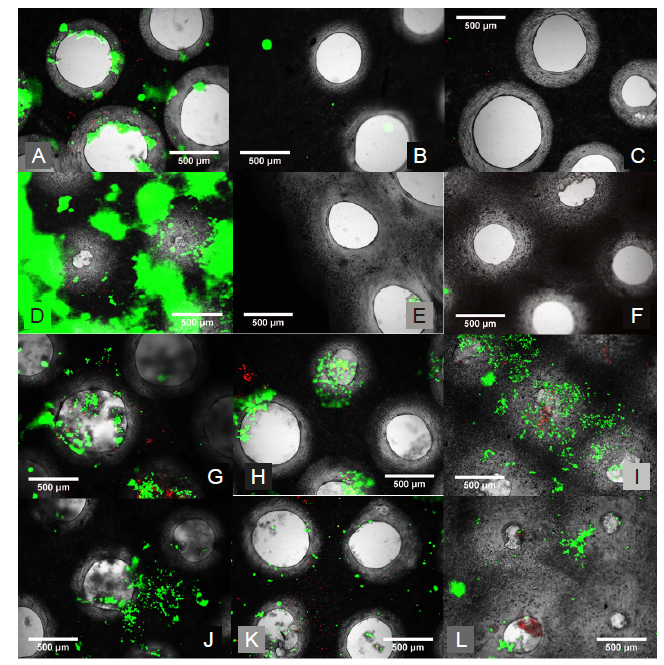
Figure 6. Live/dead staining of ADSCs on the alginate and collagen stacks. (A–F) Inverted epifluorescent microscope images after live/dead staining of ADSCs on the alginate stacks. (A) Slice 1 (top slice) showing some good cell viability on day 1. (B) Slice 2 (middle slice) showing very limited cell presence indicating no cell mobility through the construct on day 1. (C) Slice 3 (bottom slice) showing no cells and thus no mobility through the cross-linked alginate on day 3. (D) Slice 1 (top slice) showing good cell viability with some stain intake by the alginate on day 7. (E) Slice 2 (middle slice) showing limited cell viability and thus mobility on day 7. (F) Slice 3 (bottom slice) showing no cell viability and thus no mobility through the alginate even on day 7. (G–L) inverted epifluorescent microscope images after live/dead staining of ADSCs on the collagen stacks. (G) Slice 1 (top slice) showing good cell viability on day 1. (H) Slice 2 (middle slice) showing good cell viability and therefore cells must be able to travel through on day 1. (I) Slice 3 (bottom slice) showing good cell viability and thus demonstrating the mobility of cells through the collagen and the micromachined fibre mesh even after 1 day. (J) Slice 1 (top slice) showing good cell viability on day 4. (K) Slice 2 (middle slice) showing good cell viability and therefore cells must be able to travel through on day 4. (L) Slice 3 (bottom slice) showing good cell viability and thus demonstrating the mobility of cells through the collagen and the micromachined fibre mesh on day 4. Scale bars: 500 μm. ADSC: adipose derived stem cell.
| Shape | Expected height (mm) | Actual height (mm) | Ratio |
|---|---|---|---|
| Cube | 18.00 | 24.77 | 1.376 |
| Hemisphere | 12.60 | 16.87 | 1.350 |
| Pyramid | 15.40 | 21.62 | 1.404 |
Table 1. Actual versus the predicted height of the cube, pyramid and hemisphere, along with the ratio of the discrepancy
| Shape | Expected height (mm) | Actual height (mm) | Ratio |
|---|---|---|---|
| Cube | 18.00 | 24.77 | 1.376 |
| Hemisphere | 12.60 | 16.87 | 1.350 |
| Pyramid | 15.40 | 21.62 | 1.404 |
| 1. |
Templer, J.; Renner, G. J. Injuries of the external ear. Otolaryngol Clin North Am. 1990, 23, 1003-1018.
doi: 10.1016/S0030-6665(20)31223-8 URL |
| 2. |
Harris, J.; Källén, B.; Robert, E. The epidemiology of anotia and microtia. J Med Genet. 1996, 33, 809-813.
doi: 10.1136/jmg.33.10.809 URL |
| 3. |
Gassner, R.; Tuli, T.; Hächl, O.; Rudisch, A.; Ulmer, H. Cranio-maxillofacial trauma: a 10 year review of 9,543 cases with 21,067 injuries. J Craniomaxillofac Surg. 2003, 31, 51-61.
doi: 10.1016/S1010-5182(02)00168-3 URL |
| 4. |
Subhashraj, K.; Nandakumar, N.; Ravindran, C. Review of maxillofacial injuries in Chennai, India: a study of 2748 cases. Br J Oral Maxillofac Surg. 2007, 45, 637-639.
doi: 10.1016/j.bjoms.2007.03.012 URL |
| 5. | Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018, 3, 278-314. |
| 6. |
Xu, L.; Qin, H.; Tan, J.; Cheng, Z.; Luo, X.; Tan, H.; Huang, W. Clinical study of 3D printed personalized prosthesis in the treatment of bone defect after pelvic tumor resection. J Orthop Translat. 2021, 29, 163-169.
doi: 10.1016/j.jot.2021.05.007 URL |
| 7. | Pu, F.; Wu, W.; Jing, D.; Yu, Y.; Peng, Y.; Liu, J.; Wu, Q.; Wang, B.; Zhang, Z.; Shao, Z. Three-dimensional-printed titanium prostheses with bone trabeculae enable mechanical-biological reconstruction after resection of bone tumours. Biomater Transl. 2022, 3, 134-141. |
| 8. | Long, J.; Teng, B.; Zhang, W.; Li, L.; Zhang, M.; Chen, Y.; Yao, Z.; Meng, X.; Wang, X.; Qin, L.; Lai, Y. Preclinical evaluation of acute systemic toxicity of magnesium incorporated poly(lactic-co-glycolic acid) porous scaffolds by three-dimensional printing. Biomater Transl. 2021, 2, 272-284. |
| 9. |
Luquetti, D. V.; Leoncini, E.; Mastroiacovo, P. Microtia-anotia: a global review of prevalence rates. Birth Defects Res A Clin Mol Teratol. 2011, 91, 813-822.
doi: 10.1002/bdra.20836 URL |
| 10. | Duscher, D.; Shiffman, M. A. Regenerative medicine and plastic surgery. Springer Nature Switzerland: Cham, Switzerland, 2019. |
| 11. | Posnick, J. C. 18 - Grafts frequently used during orthognathic surgery and for adjunctive procedures. In Orthognathic surgery, Posnick, J. C., ed. W.B. Saunders: St. Louis, 2014; pp 607-639. |
| 12. |
Elsalanty, M. E.; Genecov, D. G. Bone grafts in craniofacial surgery. Craniomaxillofac Trauma Reconstr. 2009, 2, 125-134.
doi: 10.1055/s-0029-1215875 URL |
| 13. | Pacifici, L.; F, D. E. A.; Orefici, A.; Cielo, A. Metals used in maxillofacial surgery. Oral Implantol (Rome). 2016, 9, 107-111. |
| 14. |
Sun, H.; Guo, Q.; Shi, C.; McWilliam, R. H.; Chen, J.; Zhu, C.; Han, F.; Zhou, P.; Yang, H.; Liu, J.; Sun, X.; Meng, B.; Shu, W.; Li, B. CD271 antibody-functionalized microspheres capable of selective recruitment of reparative endogenous stem cells for in situ bone regeneration. Biomaterials. 2022, 280, 121243.
doi: 10.1016/j.biomaterials.2021.121243 URL |
| 15. |
Yang, T.; Tamaddon, M.; Jiang, L.; Wang, J.; Liu, Z.; Liu, Z.; Meng, H.; Hu, Y.; Gao, J.; Yang, X.; Zhao, Y.; Wang, Y.; Wang, A.; Wu, Q.; Liu, C.; Peng, J.; Sun, X.; Xue, Q. Bilayered scaffold with 3D printed stiff subchondral bony compartment to provide constant mechanical support for long-term cartilage regeneration. J Orthop Translat. 2021, 30, 112-121.
doi: 10.1016/j.jot.2021.09.001 URL |
| 16. |
Yin, H. W.; Feng, J. T.; Yu, B. F.; Shen, Y. D.; Gu, Y. D.; Xu, W. D. 3D printing-assisted percutaneous fixation makes the surgery for scaphoid nonunion more accurate and less invasive. J Orthop Translat. 2020, 24, 138-143.
doi: 10.1016/j.jot.2020.01.007 URL |
| 17. |
Li, D.; Xia, Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004, 16, 1151-1170.
doi: 10.1002/(ISSN)1521-4095 URL |
| 18. |
Frenot, A.; Chronakis, I. S. Polymer nanofibers assembled by electrospinning. Curr Opin Colloid Interface Sci. 2003, 8, 64-75.
doi: 10.1016/S1359-0294(03)00004-9 URL |
| 19. |
Tuzlakoglu, K.; Bolgen, N.; Salgado, A. J.; Gomes, M. E.; Piskin, E.; Reis, R. L. Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci Mater Med. 2005, 16, 1099-1104.
doi: 10.1007/s10856-005-4713-8 URL |
| 20. |
Sun, B.; Long, Y. Z.; Zhang, H. D.; Li, M. M.; Duvail, J. L.; Jiang, X. Y.; Yin, H. L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog Polym Sci. 2014, 39, 862-890.
doi: 10.1016/j.progpolymsci.2013.06.002 URL |
| 21. |
Brown, T. D.; Dalton, P. D.; Hutmacher, D. W. Melt electrospinning today: an opportune time for an emerging polymer process. Prog Polym Sci. 2016, 56, 116-166.
doi: 10.1016/j.progpolymsci.2016.01.001 URL |
| 22. | Nayak, R.; Padhye, R.; Arnold, L. 2 - Melt-electrospinning of nanofibers. In Electrospun nanofibers, Afshari, M., ed. Woodhead Publishing: 2017; pp 11-40. |
| 23. |
Zhang, L. H.; Duan, X. P.; Yan, X.; Yu, M.; Ning, X.; Zhao, Y.; Long, Y. Z. Recent advances in melt electrospinning. RSC Adv. 2016, 6, 53400-53414.
doi: 10.1039/C6RA09558E URL |
| 24. |
Brown, T. D.; Edin, F.; Detta, N.; Skelton, A. D.; Hutmacher, D. W.; Dalton, P. D. Melt electrospinning of poly(ε-caprolactone) scaffolds: phenomenological observations associated with collection and direct writing. Mater Sci Eng C Mater Biol Appl. 2014, 45, 698-708.
doi: 10.1016/j.msec.2014.07.034 URL |
| 25. |
Sun, W.; Starly, B.; Daly, A. C.; Burdick, J. A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M.; Jang, J.; Cho, D. W.; Nie, M.; Takeuchi, S.; Ostrovidov, S.; Khademhosseini, A.; Kamm, R. D.; Mironov, V.; Moroni, L.; Ozbolat, I. T. The bioprinting roadmap. Biofabrication. 2020, 12, 022002.
doi: 10.1088/1758-5090/ab5158 |
| 26. |
Holmes, A. M.; Charlton, A.; Derby, B.; Ewart, L.; Scott, A.; Shu, W. Rising to the challenge: applying biofabrication approaches for better drug and chemical product development. Biofabrication. 2017, 9, 033001.
doi: 10.1088/1758-5090/aa7bbd URL |
| 27. | Holland, I.; Logan, J.; Shi, J.; McCormick, C.; Liu, D.; Shu, W. 3D biofabrication for tubular tissue engineering. Biodes Manuf. 2018, 1, 89-100. |
| 28. | Cornelissen, D. J.; Faulkner-Jones, A.; Shu, W. Current developments in 3D bioprinting for tissue engineering. Curr Opin Biomed Eng. 2017, 2, 76-82. |
| 29. | Sahranavard, M.; Sarkari, S.; Safavi, S.; Ghorbani, F. Three-dimensional bio-printing of decellularized extracellular matrix-based bio-inks for cartilage regeneration: a systematic review. Biomater Transl. 2022, 3, 105-115. |
| 30. |
Turnbull, G.; Clarke, J.; Picard, F.; Zhang, W.; Riches, P.; Li, B.; Shu, W. 3D biofabrication for soft tissue and cartilage engineering. Med Eng Phys. 2020, 82, 13-39.
doi: 10.1016/j.medengphy.2020.06.003 URL |
| 31. |
Groll, J.; Boland, T.; Blunk, T.; Burdick, J. A.; Cho, D. W.; Dalton, P. D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V. A.; Moroni, L.; Nakamura, M.; Shu, W.; Takeuchi, S.; Vozzi, G.; Woodfield, T. B.; Xu, T.; Yoo, J. J.; Malda, J. Biofabrication: reappraising the definition of an evolving field. Biofabrication. 2016, 8, 013001.
doi: 10.1088/1758-5090/8/1/013001 URL |
| 32. |
Kang, H. W.; Lee, S. J.; Ko, I. K.; Kengla, C.; Yoo, J. J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016, 34, 312-319.
doi: 10.1038/nbt.3413 |
| 33. |
Murphy, S. V.; Atala, A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014, 32, 773-785.
doi: 10.1038/nbt.2958 |
| 34. |
Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep. 2016, 6, 24474.
doi: 10.1038/srep24474 |
| 35. |
Yu, Y.; Hua, S.; Yang, M.; Fu, Z.; Teng, S.; Niu, K.; Zhao, Q.; Yi, C. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv. 2016, 6, 110557-110565.
doi: 10.1039/C6RA17718B URL |
| 36. |
Gao, Q.; Gu, H.; Zhao, P.; Zhang, C.; Cao, M.; Fu, J.; He, Y. Fabrication of electrospun nanofibrous scaffolds with 3D controllable geometric shapes. Mater Des. 2018, 157, 159-169.
doi: 10.1016/j.matdes.2018.07.042 URL |
| 37. |
Gao, Q.; Zhao, P.; Zhou, R.; Wang, P.; Fu, J.; He, Y. Rapid assembling organ prototypes with controllable cell-laden multi-scale sheets. Bio-des Manuf. 2019, 2, 1-9.
doi: 10.1007/s42242-019-00032-z |
| 38. |
Moroni, L.; Burdick, J. A.; Highley, C.; Lee, S. J.; Morimoto, Y.; Takeuchi, S.; Yoo, J. J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater. 2018, 3, 21-37.
doi: 10.1038/s41578-018-0006-y |
| 39. | Dalton, P. D.; Woodfield, T. B. F.; Mironov, V.; Groll, J. Advances in hybrid fabrication toward hierarchical tissue constructs. Adv Sci (Weinh). 2020, 7, 1902953. |
| 40. | Sankar, S.; Sharma, C. S.; Rath, S. N.; Ramakrishna, S. Electrospun nanofibres to mimic natural hierarchical structure of tissues: application in musculoskeletal regeneration. J Tissue Eng Regen Med. 2018, 12, e604-e619. |
| 41. |
Kong, B.; Sun, W.; Chen, G.; Tang, S.; Li, M.; Shao, Z.; Mi, S. Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci Rep. 2017, 7, 970.
doi: 10.1038/s41598-017-01072-0 |
| 42. |
Lee, B. L.; Jeon, H.; Wang, A.; Yan, Z.; Yu, J.; Grigoropoulos, C.; Li, S. Femtosecond laser ablation enhances cell infiltration into three-dimensional electrospun scaffolds. Acta Biomater. 2012, 8, 2648-2658.
doi: 10.1016/j.actbio.2012.04.023 URL |
| 43. |
Aquino-Martínez, R.; Angelo, A. P.; Pujol, F. V. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem Cell Res Ther. 2017, 8, 265.
doi: 10.1186/s13287-017-0713-0 URL |
| 44. | Laco, F.; Grant, M. H.; Black, R. A. Collagen-nanofiber hydrogel composites promote contact guidance of human lymphatic microvascular endothelial cells and directed capillary tube formation. J Biomed Mater Res A. 2013, 101, 1787-1799. |
| 45. |
Li, H.; Cheng, F.; Robledo-Lara, J. A.; Liao, J.; Wang, Z.; Zhang, Y. S. Fabrication of paper-based devices for in vitro tissue modeling. Bio-des Manuf. 2020, 3, 252-265.
doi: 10.1007/s42242-020-00077-5 |
| 46. |
Moe, S. T.; Skjaak-Braek, G.; Elgsaeter, A.; Smidsroed, O. Swelling of covalently crosslinked alginate gels: influence of ionic solutes and nonpolar solvents. Macromolecules. 1993, 26, 3589-3597.
doi: 10.1021/ma00066a017 URL |
| 47. | No authors listed. Rapid analysis of human adipose-derived stem cells and 3T3-L1 differentiation towards adipocytes using the ScepterTM 2.0 Cell Counter. Biotechniques. 2012, 53, 2. |
| 48. |
Laurencin, C. T.; Ambrosio, A. M.; Borden, M. D.; Cooper, J. A., Jr. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999, 1, 19-46.
doi: 10.1146/bioeng.1999.1.issue-1 URL |
| 49. |
Bridle, H.; Wang, W.; Gavriilidou, D.; Amalou, F.; Hand, D. P.; Shu, W. Static mode microfluidic cantilevers for detection of waterborne pathogens. Sens Actuators A Phys. 2016, 247, 144-149.
doi: 10.1016/j.sna.2016.05.011 URL |
| 50. |
Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D. J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015, 7, 044102.
doi: 10.1088/1758-5090/7/4/044102 URL |
| [1] | Ahlam A. Abdalla, Catherine J. Pendegrass. Biological approaches to the repair and regeneration of the rotator cuff tendon-bone enthesis: a literature review [J]. Biomaterials Translational, 2023, 4(2): 85-103. |
| [2] | Rob Jess, Tao Ling, Yi Xiong, Chris J. Wright, Feihu Zhao. Mechanical environment for in vitro cartilage tissue engineering assisted by in silico models [J]. Biomaterials Translational, 2023, 4(1): 18-26. |
| [3] | Chavee Laomeephol, Helena Ferreira, Sorada Kanokpanont, Jittima Amie Luckanagul, Nuno M Neves, Siriporn Damrongsakkul. Osteogenic differentiation of encapsulated cells in dexamethasone–loaded phospholipid–induced silk fibroin hydrogels [J]. Biomaterials Translational, 2022, 3(3): 213-220. |
| [4] | Ricardo Donate, Maryam Tamaddon, Viviana Ribeiro, Mario Monzón, J. Miguel Oliveira, Chaozong Liu. Translation through collaboration: practice applied in BAMOS project in in vivo testing of innovative osteochondral scaffolds [J]. Biomaterials Translational, 2022, 3(2): 102-104. |
| [5] | Melika Sahranavard, Soulmaz Sarkari, SeyedehMina Safavi, Farnaz Ghorbani. Three-dimensional bio-printing of decellularized extracellular matrix-based bio-inks for cartilage regeneration: a systematic review [J]. Biomaterials Translational, 2022, 3(2): 105-115. |
| [6] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [7] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [8] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [9] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [10] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [11] | Maryam Tamaddon, Helena Gilja, Ling Wang, J. Miguel Oliveira, Xiaodan Sun, Rongwei Tan, Chaozong Liu. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic [J]. Biomaterials Translational, 2020, 1(1): 3-17. |
| [12] | Xing Yang, Yuanyuan Li, Xujie Liu, Wei He, Qianli Huang, Qingling Feng. Nanoparticles and their effects on differentiation of mesenchymal stem cells [J]. Biomaterials Translational, 2020, 1(1): 58-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||