Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (2): 85-103.doi: 10.12336/biomatertransl.2023.02.004
• REVIEW • Previous Articles Next Articles
Ahlam A. Abdalla ), Catherine J. Pendegrass
), Catherine J. Pendegrass )
)
Received:2023-02-04
Revised:2023-03-15
Accepted:2023-05-05
Online:2023-06-28
Published:2023-06-28
Contact:
Ahlam A. Abdalla,Catherine J. Pendegrass
E-mail:ahlam.abdalla.21@ucl.ac.uk;c.pendegrass@ucl.ac.uk
About author:Catherine J. Pendegrass, c.pendegrass@ucl.ac.uk.†Present Addresses: Department of Orthopaedics & Musculoskeletal Science, Division of Surgery & Interventional Sciences, University College London, Brockley Hill, Stanmore, UK
| Author | Study | Model | Tear | Intervention | Outcome measure | Results |
|---|---|---|---|---|---|---|
| Honda et al. | In-vivo | Rabbit | Chronic full | MSC + HA | Biomechanical, histological, and immunohistochemical analyses | Positive: Improved ultimate load and faster healing |
| Jo et al. | Clinical | Human | Chronic full | BMSC + arthroscopic repeated channeling | Pain scale, ROM, muscle strength, patient satisfaction questionnaire, and functional scores. Structural integrity by MRI and CT | Positive: Enhanced structural integrity of the repair and decreased retears |
| Hernigou et al. | Clinical | Human | Chronic partial | BMSC + arthroscopy | MRI | Positive: Faster complete healing/retear prevention |
| Taniguchi et al. | Clinical | Human | Chronic partial | ASH + BMSC | MRI | Positive: Reduced retear rates/better integrity |
| Han et al. | In-vitro, in-vivo | Rat | Acute full | PRP-infused BMSC | Expression of genes that related to tissue repair, bone formation, and tendon regeneration; | Positive: Stronger signals to angiogenesis, bone formation, and tendon generation in-situ |
| Biomechanical assessment | Promoted healing in-vivo | |||||
| Gulotta et al. | In-vivo | Rat | Acute full | BMSC | Biomechanical & histological analyses | Negative: No change in structure, composition, or strength |
| Gulotta et al. | In-vivo | Rat | Acute full | MT1-MMP-transduced MSCs | Biomechanical & histological analyses | Positive: Better fibrocartilage formation, higher ultimate load and stress to failure, and higher stiffness |
| Oh et al. | In-vivo | Rabbit | Chronic full | ADSC + suture | Electromyographic, biomechanical & histological analyses | Positive: Larger load to failure and less fat infiltration |
| Chen et al. | In-vivo | Murine | Acute full | ADSC imbedded in fibrin sealant scaffold | Biomechanical & histological analyses | Positive: Better biomechanical strength and histological score |
| Choi et al. | In-vivo | Rat | Chronic full | ADSC sheets interposed at the enthesis | Biomechanical & histological analyses | Positive: Successful complete regeneration and biomechanical strength |
| Valencia Mora et al. | In-vivo | Rat | Chronic full | ADSC, ADSC + TGF-β3 | Biomechanical & histological analyses | Positive: Reduced inflammation |
| Negative: Unchanged maximum load, elastic energy, mechanical deformation, and stiffness |
Table 1. Summary of cell-based therapies literature findings
| Author | Study | Model | Tear | Intervention | Outcome measure | Results |
|---|---|---|---|---|---|---|
| Honda et al. | In-vivo | Rabbit | Chronic full | MSC + HA | Biomechanical, histological, and immunohistochemical analyses | Positive: Improved ultimate load and faster healing |
| Jo et al. | Clinical | Human | Chronic full | BMSC + arthroscopic repeated channeling | Pain scale, ROM, muscle strength, patient satisfaction questionnaire, and functional scores. Structural integrity by MRI and CT | Positive: Enhanced structural integrity of the repair and decreased retears |
| Hernigou et al. | Clinical | Human | Chronic partial | BMSC + arthroscopy | MRI | Positive: Faster complete healing/retear prevention |
| Taniguchi et al. | Clinical | Human | Chronic partial | ASH + BMSC | MRI | Positive: Reduced retear rates/better integrity |
| Han et al. | In-vitro, in-vivo | Rat | Acute full | PRP-infused BMSC | Expression of genes that related to tissue repair, bone formation, and tendon regeneration; | Positive: Stronger signals to angiogenesis, bone formation, and tendon generation in-situ |
| Biomechanical assessment | Promoted healing in-vivo | |||||
| Gulotta et al. | In-vivo | Rat | Acute full | BMSC | Biomechanical & histological analyses | Negative: No change in structure, composition, or strength |
| Gulotta et al. | In-vivo | Rat | Acute full | MT1-MMP-transduced MSCs | Biomechanical & histological analyses | Positive: Better fibrocartilage formation, higher ultimate load and stress to failure, and higher stiffness |
| Oh et al. | In-vivo | Rabbit | Chronic full | ADSC + suture | Electromyographic, biomechanical & histological analyses | Positive: Larger load to failure and less fat infiltration |
| Chen et al. | In-vivo | Murine | Acute full | ADSC imbedded in fibrin sealant scaffold | Biomechanical & histological analyses | Positive: Better biomechanical strength and histological score |
| Choi et al. | In-vivo | Rat | Chronic full | ADSC sheets interposed at the enthesis | Biomechanical & histological analyses | Positive: Successful complete regeneration and biomechanical strength |
| Valencia Mora et al. | In-vivo | Rat | Chronic full | ADSC, ADSC + TGF-β3 | Biomechanical & histological analyses | Positive: Reduced inflammation |
| Negative: Unchanged maximum load, elastic energy, mechanical deformation, and stiffness |
| Author | Study | Model | Tear | Intervention | Outcome measure | Study results |
|---|---|---|---|---|---|---|
| Zong et al. | In-vivo | Rat | Acute full | Ihh + MSC | Immunohistochemical staining and proliferating cell nuclear antigen staining | Positive: Increased Gli1 and Patched1 expression. More organised and stronger staining for collagen II |
| Schwartz et al. | In-vivo | Murine | Acute partial | Ihh | Lineage tracing | Positive: Gli1 lineage cells that originate in utero eventually populate the entire mature enthesis. Ablation of the Hh-responsive cells during the first week of postnatal development resulted in a loss of mineralised fibrocartilage |
| Schwartz et al. | In-vivo | Mouse | Acute partial | Ihh | Lineage tracing | Positive: High levels of Gli1 expression in immature mice and mature entheses had fewer Gli1+ cells |
| Hettrich et al. | In-vivo | Rat | Acute full | Systemic PTH | Histologic, immunohistochemical, biomechanical analyses | Positive: Higher stiffness, bone volume and mineral content; More fibrocartilage, osteoblasts, and blood vessels formation; Better collagen orientation |
| Duchman et al. | In-vivo | Rat | Acute full | Systemic rhPTH | Biomechanical and histologic analysis | Positive: Higher load to failure. Expression of intracellular and extracellular VEGF |
| Oh et al. | Clinical | Human | Chronic full | Systemic rhPTH | MRI, ROM, American Shoulder and Elbow Surgeons and Constant scores, and simple shoulder test | Positive: Lower retear rate |
Table 2. Summary of signaling molecules therapies literature findings
| Author | Study | Model | Tear | Intervention | Outcome measure | Study results |
|---|---|---|---|---|---|---|
| Zong et al. | In-vivo | Rat | Acute full | Ihh + MSC | Immunohistochemical staining and proliferating cell nuclear antigen staining | Positive: Increased Gli1 and Patched1 expression. More organised and stronger staining for collagen II |
| Schwartz et al. | In-vivo | Murine | Acute partial | Ihh | Lineage tracing | Positive: Gli1 lineage cells that originate in utero eventually populate the entire mature enthesis. Ablation of the Hh-responsive cells during the first week of postnatal development resulted in a loss of mineralised fibrocartilage |
| Schwartz et al. | In-vivo | Mouse | Acute partial | Ihh | Lineage tracing | Positive: High levels of Gli1 expression in immature mice and mature entheses had fewer Gli1+ cells |
| Hettrich et al. | In-vivo | Rat | Acute full | Systemic PTH | Histologic, immunohistochemical, biomechanical analyses | Positive: Higher stiffness, bone volume and mineral content; More fibrocartilage, osteoblasts, and blood vessels formation; Better collagen orientation |
| Duchman et al. | In-vivo | Rat | Acute full | Systemic rhPTH | Biomechanical and histologic analysis | Positive: Higher load to failure. Expression of intracellular and extracellular VEGF |
| Oh et al. | Clinical | Human | Chronic full | Systemic rhPTH | MRI, ROM, American Shoulder and Elbow Surgeons and Constant scores, and simple shoulder test | Positive: Lower retear rate |
| Author | Study | Model | Tear | Intervention | Outcome measure | Study results |
|---|---|---|---|---|---|---|
| Würgler-Hauri et al. | In-vivo | Rat | Acute full | BMP-12-14, bFGF, COMP, CTGF, PDGFB, TGF-β1 | Immunohistochemical staining | Positive: Increase in the expression of all GFs at 1 week, and followed by a return to control or undetectable levels by 16 weeks |
| Kobayashi et al. | In-vivo | Rabbit | Acute full | BMP-12-14, bFGF, COMP, CTGF, PDGFB, TGF-β1 | Light microscopy after staining with hematoxylin-eosin and Elastica-Masson; Immunohistochemical staining | Positive: GFs are involved in early phases of healing promotion |
| Rodeo et al. | In-vivo | Sheep | Acute full | BMP and VEGF | MRI, plain radiographs, histologic analysis, and biomechanical testing | Positive: Greater formation of new bone, fibrocartilage, and soft tissue, with an increase in tendon attachment strength |
| Angeline and Rodeo | In-vivo | Sheep | Acute full | BMP and VEGF | Histologic analysis | Positive: Induced angiogenesis and vasculogenesis. Faster and better recovery |
| Manning et al. | In-vivo | Rat | Acute full | TGF-β3 | Histologic and biomechanical analyses | Negative: Disorganised scar and inferior mechanical properties |
| Kim et al. | In-vivo | Rat | Acute full | TGF-β3 | Histologic and biomechanical analyses | Negative: Disorganised scar and inferior mechanical properties |
| Davies et al. | In-vivo | Mouse | Acute full | Inhibiting TGF-β1 | Histologic analysis | Positive: Reduced fibrosis, fatty infiltration, and muscle atrophy |
| Jensen et al. | In-vivo | Mouse | Acute full and partial | TGF-β3 + cytokine + MMP inhibitors | Reviewing the literature | Positive: Enhanced healing |
| Zhou et al. | In-vivo | Rat | Acute full | rhFGF-18 | Histologic analysis | Positive: Promoted chondrogenesis and promoted healing and regeneration |
| Sitcheran et al. | In-vivo | Mouse | Acute full | TGF-β3 | Histologic analysis | Negative: No improvement in healing |
| Gulotta et al. | In-vivo | Rat | Acute and chronic full | TNF inhibitor | Histologic and biomechanical analyses | Positive: Elevated fibrocartilage, and enhanced load to failure and stiffness |
| Dorman et al. | In-vivo | Mouse | Acute full | BMP-2-7 | Histologic analysis | Positive: Fully healed enthesis without toxicity |
| Kabuto et al. | In-vivo | Rat | Acute full | BMP-2-7 | Histologic and biomechanical analyses | Positive: Improved biomechanical properties |
Table 3. Summary of growth factor-based therapies literature findings
| Author | Study | Model | Tear | Intervention | Outcome measure | Study results |
|---|---|---|---|---|---|---|
| Würgler-Hauri et al. | In-vivo | Rat | Acute full | BMP-12-14, bFGF, COMP, CTGF, PDGFB, TGF-β1 | Immunohistochemical staining | Positive: Increase in the expression of all GFs at 1 week, and followed by a return to control or undetectable levels by 16 weeks |
| Kobayashi et al. | In-vivo | Rabbit | Acute full | BMP-12-14, bFGF, COMP, CTGF, PDGFB, TGF-β1 | Light microscopy after staining with hematoxylin-eosin and Elastica-Masson; Immunohistochemical staining | Positive: GFs are involved in early phases of healing promotion |
| Rodeo et al. | In-vivo | Sheep | Acute full | BMP and VEGF | MRI, plain radiographs, histologic analysis, and biomechanical testing | Positive: Greater formation of new bone, fibrocartilage, and soft tissue, with an increase in tendon attachment strength |
| Angeline and Rodeo | In-vivo | Sheep | Acute full | BMP and VEGF | Histologic analysis | Positive: Induced angiogenesis and vasculogenesis. Faster and better recovery |
| Manning et al. | In-vivo | Rat | Acute full | TGF-β3 | Histologic and biomechanical analyses | Negative: Disorganised scar and inferior mechanical properties |
| Kim et al. | In-vivo | Rat | Acute full | TGF-β3 | Histologic and biomechanical analyses | Negative: Disorganised scar and inferior mechanical properties |
| Davies et al. | In-vivo | Mouse | Acute full | Inhibiting TGF-β1 | Histologic analysis | Positive: Reduced fibrosis, fatty infiltration, and muscle atrophy |
| Jensen et al. | In-vivo | Mouse | Acute full and partial | TGF-β3 + cytokine + MMP inhibitors | Reviewing the literature | Positive: Enhanced healing |
| Zhou et al. | In-vivo | Rat | Acute full | rhFGF-18 | Histologic analysis | Positive: Promoted chondrogenesis and promoted healing and regeneration |
| Sitcheran et al. | In-vivo | Mouse | Acute full | TGF-β3 | Histologic analysis | Negative: No improvement in healing |
| Gulotta et al. | In-vivo | Rat | Acute and chronic full | TNF inhibitor | Histologic and biomechanical analyses | Positive: Elevated fibrocartilage, and enhanced load to failure and stiffness |
| Dorman et al. | In-vivo | Mouse | Acute full | BMP-2-7 | Histologic analysis | Positive: Fully healed enthesis without toxicity |
| Kabuto et al. | In-vivo | Rat | Acute full | BMP-2-7 | Histologic and biomechanical analyses | Positive: Improved biomechanical properties |
| Author | Study | Model | Tear | Intervention | Outcome measure | Study results | ||
|---|---|---|---|---|---|---|---|---|
| Huang et al. | In-vivo | Rabbit | Acute full | KGN-loaded GelMA hydrogel + BMSC scaffold | Macroscopy, microcomputed tomography, histology, and biomechanical tests | Positive: Promoted fibrocartilage formation and superior mechanical properties | ||
| Novakova et al. | In-vivo | Sheep | Acute full | Engineered tendon construct with BMSCs | X-ray and biomechanical tests | Positive: Native-like enthesis with higher modulus | ||
| Han et al. | In-vivo | Rabbit | Acute full | BMP-2 + polyaspartic acid + Smad/RUNX2 signaling | Transmission electron microscopy staining; Biomechanics and histological assessment | Positive: Increased bone and tissue mineral density and ultimate load strength | ||
| Ousema et al. | In-vitro | RC tear | 3D woven PCL scaffold + IL-1 inhibition on MSCs | Histological, biomechanical, and immunohistochemistry analyses | Positive: Mechanical functionality preserved with the use of a 3D woven PCL scaffold | |||
| Jiang et al. | In-vitro | RC tear | 3D PLGA scaffold + a cell-laden collagen hydrogel + ADSCs | Histological and biomechanics analyses | Positive: Improvement in mechanical properties and biocompatibility | |||
| Iannotti et al. | Clinical | Human | Chronic full | SIS | Penn shoulder-score questionnaire and MRI | Negative: No improvement in healing and clinical results | ||
| Malcarney et al. | Clinical | Human | Chronic full | SIS | Study discontinued due to adverse effects | Negative: Inflammatory reaction | ||
| Sclamberg et al. | Clinical | Human | Chronic full | SIS | Patient questionnaire, MRI, and ASES | Negative: No improvement and worse pot-operative outcomes | ||
| Ciampi et al. | Clinical | Aging human | Chronic full | Polypropylene augmentation patch | Ultrasound, muscle strength, and VAS | Positive: Improved muscle strength, pain score, and tendon integrity | ||
| Cai et al. | Clinical | Aging human | Chronic full | 3D biological collagen-I mesh | MRI, VAS, UCLA SST, and Constant score | Positive: Less retear rates | ||
| Hoberman et al. | In-vitro | RC tear | DBM + BMSCs + PRP | Adhesion, proliferation, and differentiation assays | Positive: Better adhesion, proliferation, and differentiation | |||
| Thangarajah et al. | In-vivo | Rat | Chronic full | DBM | Histological analysis | Negative: No improvement in collagen organisation and fibrocartilage formation | ||
| Thangarajah et al. | In-vivo | Rat | Chronic full | DBM + MSCs | Histological analysis | Positive: Enhanced healing | ||
| Smith et al. | In-vivo | Canine | Chronic full | PRP + DBM | Histological and biomechanical analysis | Positive: Improvement in strength and histological structure | ||
| Wellington et al. | Clinical | Human | Chronic full | DBM + MSCs | MRI | Negative: Supraspinatus failure | ||
| Chae et al. | In-vivo | Mouse | Chronic full | 3D cell-printed tendon-bone interface construct | >Gait analysis, histological and biomechanical analysis | Positive: Fully formed enthesis, improved shoulder outcome and biomechanical properties | ||
| Yoon et al. | Clinical | Aging and young human | Chronic full | ADF | ROM, VAS, and MRI | Positive: No adverse effects, improved VAS, and functional scores | ||
| Warth et al. | Clinical | Human | Chronic full | PRP | MRI and Constant score | Positive: Lower retear and improved constant score | ||
| Castricini et al. | Clinical | Human | Chronic partial | Autologous PRFM | MRI | Positive: Improved tendon integrity | ||
| Chronic full | Autologous PRFM | MRI | Negative: No difference in constant score and tendon integrity | |||||
| Giovannetti de Sanctis et al. | Systematic review | Human | Chronic partial | PRP | Shoulder function and VAS | Positive: Improved pain and shoulder function | ||
| Von Wehren et al. | Clinical | Human | Chronic partial | PRP | MRI, Constant score, ASES, shoulder ROM, and VAS | Positive: Improved pain and function | ||
| Xu and Xue | Systematic review | Human | Chronic full | PRP | Retear rate, Constant, UCLA, ASES, VAS, and adverse effects | Positive: Improved shoulder outcome and reduced retear rate | ||
| Rha et al. | Clinical | Human | Chronic partial | PRP | Shoulder Pain and Disability Index, ROM, and ultrasound | Positive: No adverse effects, and improved shoulder pain and function | ||
| Shams et al. | Clinical | Human | Chronic partial | PRP | MRI, ASES, Constant Score, SST, and VAS | Positive: Improved shoulder function and minor MRI improvement | ||
| Cai et al. | Clinical | Young human | Acute partial | SH + PRP | VAS, Constant score, and MRI | Positive: Better VAS, constant score, and MRI findings | ||
| Ryan et al. | Systematic review | Human | Chronic full | PRP | Constant, ASES, UCLA, SST, VAS, and retear rate | Positive: Reduced retear rates and improved clinical outcomes | ||
| Lavoie-Gagne et al. | Systematic review and meta-analysis | Human | Chronic full | PRP | Clinical characteristics, retear rates, ROM, and patient reported outcomes | Positive: Reduced retear rates and improved clinical outcomes | ||
| Ilhanli et al. | Clinical | Human | Chronic partial | PRP | ROM, VAS, Disabilities of Arm, Shoulder and Hand questionnaire, Neer's, Hawkins' and drop arm tests and Beck Depression Inventory questionnaire | Negative: Results were not superior to physiotherapy | ||
Table 4. Summary of scaffold-based therapies literature findings
| Author | Study | Model | Tear | Intervention | Outcome measure | Study results | ||
|---|---|---|---|---|---|---|---|---|
| Huang et al. | In-vivo | Rabbit | Acute full | KGN-loaded GelMA hydrogel + BMSC scaffold | Macroscopy, microcomputed tomography, histology, and biomechanical tests | Positive: Promoted fibrocartilage formation and superior mechanical properties | ||
| Novakova et al. | In-vivo | Sheep | Acute full | Engineered tendon construct with BMSCs | X-ray and biomechanical tests | Positive: Native-like enthesis with higher modulus | ||
| Han et al. | In-vivo | Rabbit | Acute full | BMP-2 + polyaspartic acid + Smad/RUNX2 signaling | Transmission electron microscopy staining; Biomechanics and histological assessment | Positive: Increased bone and tissue mineral density and ultimate load strength | ||
| Ousema et al. | In-vitro | RC tear | 3D woven PCL scaffold + IL-1 inhibition on MSCs | Histological, biomechanical, and immunohistochemistry analyses | Positive: Mechanical functionality preserved with the use of a 3D woven PCL scaffold | |||
| Jiang et al. | In-vitro | RC tear | 3D PLGA scaffold + a cell-laden collagen hydrogel + ADSCs | Histological and biomechanics analyses | Positive: Improvement in mechanical properties and biocompatibility | |||
| Iannotti et al. | Clinical | Human | Chronic full | SIS | Penn shoulder-score questionnaire and MRI | Negative: No improvement in healing and clinical results | ||
| Malcarney et al. | Clinical | Human | Chronic full | SIS | Study discontinued due to adverse effects | Negative: Inflammatory reaction | ||
| Sclamberg et al. | Clinical | Human | Chronic full | SIS | Patient questionnaire, MRI, and ASES | Negative: No improvement and worse pot-operative outcomes | ||
| Ciampi et al. | Clinical | Aging human | Chronic full | Polypropylene augmentation patch | Ultrasound, muscle strength, and VAS | Positive: Improved muscle strength, pain score, and tendon integrity | ||
| Cai et al. | Clinical | Aging human | Chronic full | 3D biological collagen-I mesh | MRI, VAS, UCLA SST, and Constant score | Positive: Less retear rates | ||
| Hoberman et al. | In-vitro | RC tear | DBM + BMSCs + PRP | Adhesion, proliferation, and differentiation assays | Positive: Better adhesion, proliferation, and differentiation | |||
| Thangarajah et al. | In-vivo | Rat | Chronic full | DBM | Histological analysis | Negative: No improvement in collagen organisation and fibrocartilage formation | ||
| Thangarajah et al. | In-vivo | Rat | Chronic full | DBM + MSCs | Histological analysis | Positive: Enhanced healing | ||
| Smith et al. | In-vivo | Canine | Chronic full | PRP + DBM | Histological and biomechanical analysis | Positive: Improvement in strength and histological structure | ||
| Wellington et al. | Clinical | Human | Chronic full | DBM + MSCs | MRI | Negative: Supraspinatus failure | ||
| Chae et al. | In-vivo | Mouse | Chronic full | 3D cell-printed tendon-bone interface construct | >Gait analysis, histological and biomechanical analysis | Positive: Fully formed enthesis, improved shoulder outcome and biomechanical properties | ||
| Yoon et al. | Clinical | Aging and young human | Chronic full | ADF | ROM, VAS, and MRI | Positive: No adverse effects, improved VAS, and functional scores | ||
| Warth et al. | Clinical | Human | Chronic full | PRP | MRI and Constant score | Positive: Lower retear and improved constant score | ||
| Castricini et al. | Clinical | Human | Chronic partial | Autologous PRFM | MRI | Positive: Improved tendon integrity | ||
| Chronic full | Autologous PRFM | MRI | Negative: No difference in constant score and tendon integrity | |||||
| Giovannetti de Sanctis et al. | Systematic review | Human | Chronic partial | PRP | Shoulder function and VAS | Positive: Improved pain and shoulder function | ||
| Von Wehren et al. | Clinical | Human | Chronic partial | PRP | MRI, Constant score, ASES, shoulder ROM, and VAS | Positive: Improved pain and function | ||
| Xu and Xue | Systematic review | Human | Chronic full | PRP | Retear rate, Constant, UCLA, ASES, VAS, and adverse effects | Positive: Improved shoulder outcome and reduced retear rate | ||
| Rha et al. | Clinical | Human | Chronic partial | PRP | Shoulder Pain and Disability Index, ROM, and ultrasound | Positive: No adverse effects, and improved shoulder pain and function | ||
| Shams et al. | Clinical | Human | Chronic partial | PRP | MRI, ASES, Constant Score, SST, and VAS | Positive: Improved shoulder function and minor MRI improvement | ||
| Cai et al. | Clinical | Young human | Acute partial | SH + PRP | VAS, Constant score, and MRI | Positive: Better VAS, constant score, and MRI findings | ||
| Ryan et al. | Systematic review | Human | Chronic full | PRP | Constant, ASES, UCLA, SST, VAS, and retear rate | Positive: Reduced retear rates and improved clinical outcomes | ||
| Lavoie-Gagne et al. | Systematic review and meta-analysis | Human | Chronic full | PRP | Clinical characteristics, retear rates, ROM, and patient reported outcomes | Positive: Reduced retear rates and improved clinical outcomes | ||
| Ilhanli et al. | Clinical | Human | Chronic partial | PRP | ROM, VAS, Disabilities of Arm, Shoulder and Hand questionnaire, Neer's, Hawkins' and drop arm tests and Beck Depression Inventory questionnaire | Negative: Results were not superior to physiotherapy | ||
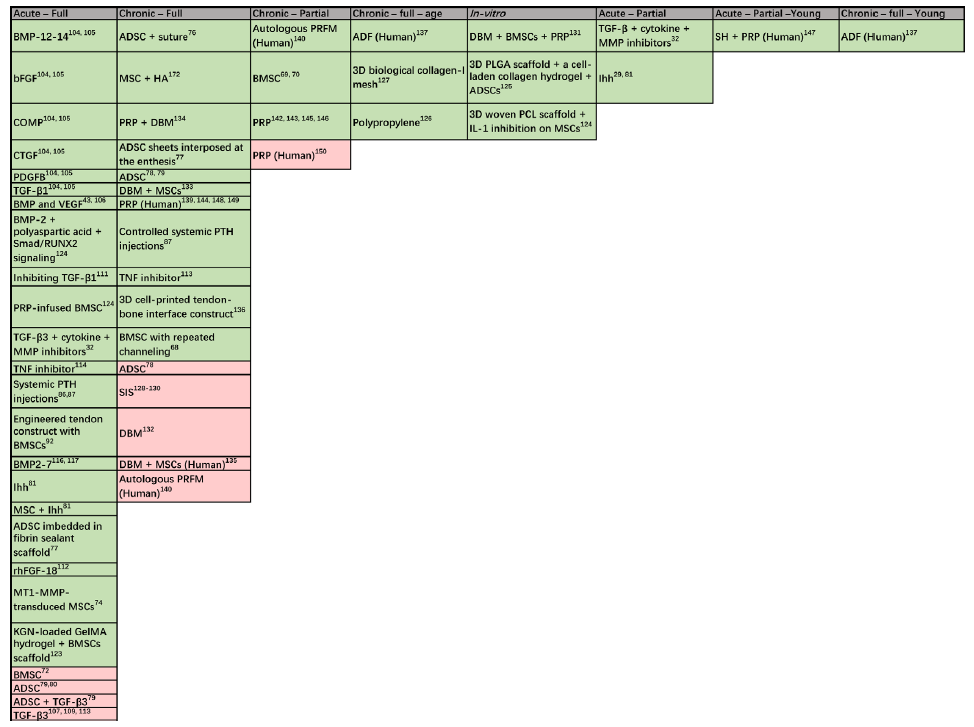
Figure 2. Categorised literature findings: stem cells, GFs, and scaffolds vary in effect depending on the extent of the tear, chronicity level, population, and their combinations. Majority of studies investigated acute full tears, while less studies were investigating partial and full chronic tears. Very few studies considered the age of the studied population. 3D: three-dimensional; ADF: autologous dermal fibroblast; ADSC: adipose-derived stem cell; bFGF: basic fibroblast growth factor; BMP: bone morphogenetic protein; BMSC: bone marrow mesenchymal stem cell; COMP: cartilage oligomeric matrix protein; CTGF: connective tissue growth factor; DBM: demineralised bone matrix; GelMA: gelatin methacrylol; GF: growth factor; HA: hyaluronic acid; Ihh: Indian hedgehog; KGN: kartogenin; MMP: matrix metalloproteinase; MSC: mesenchymal stem cell; PCL: polycaprolactone; PDGFB: platelet-derived growth factor-B; PLGA: polylactide-co-glycolide acid; PRFM: platelet-rich fibrin clot matrix; PRP: platelet-rich plasma; PTH: parathyroid hormone; SH: sodium hyaluronate; SIS: small intestine submucosa; TGF: transforming growth factor; TNF: tumour necrosis factor.
| 1. |
Liu, Y. X.; Thomopoulos, S.; Birman, V.; Li, J. S.; Genin, G. M. Bi-material attachment through a compliant interfacial system at the tendon-to-bone insertion site. Mech Mater. 2012, 44, 10.1016/j.mechmat.2011.1008.1005.
doi: 10.1016/j.mechmat.2011.1008.1005 |
| 2. |
Thomopoulos, S.; Williams, G. R.; Gimbel, J. A.; Favata, M.; Soslowsky, L. J. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003, 21, 413-419.
doi: 10.1016/S0736-0266(03)0057-3 URL |
| 3. |
Shaw, H. M.; Benjamin, M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand J Med Sci Sports. 2007, 17, 303-315.
doi: 10.1111/j.1600-0838.2007.00689.x URL |
| 4. |
Lu, H. H.; Thomopoulos, S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013, 15, 201-226.
doi: 10.1146/bioeng.2013.15.issue-1 URL |
| 5. | Milgrom, C.; Schaffler, M.; Gilbert, S.; van Holsbeeck, M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995, 77, 296-298. |
| 6. |
Yamaguchi, K.; Ditsios, K.; Middleton, W. D.; Hildebolt, C. F.; Galatz, L. M.; Teefey, S. A. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006, 88, 1699-1704.
doi: 10.2106/JBJS.E.00835 URL |
| 7. |
Galatz, L. M.; Ball, C. M.; Teefey, S. A.; Middleton, W. D.; Yamaguchi, K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004, 86, 219-224.
doi: 10.2106/00004623-200402000-00002 URL |
| 8. |
Shindle, M. K.; Chen, C. C.; Robertson, C.; DiTullio, A. E.; Paulus, M. C.; Clinton, C. M.; Cordasco, F. A.; Rodeo, S. A.; Warren, R. F. Full-thickness supraspinatus tears are associated with more synovial inflammation and tissue degeneration than partial-thickness tears. J Shoulder Elbow Surg. 2011, 20, 917-927.
doi: 10.1016/j.jse.2011.02.015 URL |
| 9. | Benjamin, M.; Qin, S.; Ralphs, J. R. Fibrocartilage associated with human tendons and their pulleys. J Anat. 1995, 187 (Pt 3), 625-633. |
| 10. |
Benjamin, M.; Kumai, T.; Milz, S.; Boszczyk, B. M.; Boszczyk, A. A.; Ralphs, J. R. The skeletal attachment of tendons--tendon “entheses”. Comp Biochem Physiol A Mol Integr Physiol. 2002, 133, 931-945.
doi: 10.1016/S1095-6433(02)00138-1 URL |
| 11. | Frowen, P.; Benjamin, M. Variations in the quality of uncalcified fibrocartilage at the insertions of the extrinsic calf muscles in the foot. J Anat. 1995, 186 (Pt 2), 417-421. |
| 12. |
Matyas, J. R.; Anton, M. G.; Shrive, N. G.; Frank, C. B. Stress governs tissue phenotype at the femoral insertion of the rabbit MCL. J Biomech. 1995, 28, 147-157.
doi: 10.1016/0021-9290(94)00058-C URL |
| 13. |
Yang, P. J.; Temenoff, J. S. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009, 15, 127-141.
doi: 10.1089/ten.teb.2008.0371 URL |
| 14. | Benjamin, M.; Ralphs, J. R. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J Anat. 1998, 193 (Pt 4), 481-494. |
| 15. |
Connizzo, B. K.; Adams, S. M.; Adams, T. H.; Jawad, A. F.; Birk, D. E.; Soslowsky, L. J. Multiscale regression modeling in mouse supraspinatus tendons reveals that dynamic processes act as mediators in structure-function relationships. J Biomech. 2016, 49, 1649-1657.
doi: 10.1016/j.jbiomech.2016.03.053 URL |
| 16. |
Schwartz, A. G.; Lipner, J. H.; Pasteris, J. D.; Genin, G. M.; Thomopoulos, S. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone. 2013, 55, 44-51.
doi: 10.1016/j.bone.2013.03.010 URL |
| 17. |
Knese, K. H.; Biermann, H. Osteogenesis in tendon and ligament insertions in the area of the original chondral apophyses. Z Zellforsch Mikrosk Anat. 1958, 49, 142-187.
doi: 10.1007/BF00324425 URL |
| 18. |
Thomopoulos, S.; Hattersley, G.; Rosen, V.; Mertens, M.; Galatz, L.; Williams, G. R.; Soslowsky, L. J. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002, 20, 454-463.
doi: 10.1016/S0736-0266(01)00144-9 URL |
| 19. |
Galatz, L. M.; Sandell, L. J.; Rothermich, S. Y.; Das, R.; Mastny, A.; Havlioglu, N.; Silva, M. J.; Thomopoulos, S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006, 24, 541-550.
doi: 10.1002/(ISSN)1554-527X URL |
| 20. | Ralphs, J. R.; Tyers, R. N.; Benjamin, M. Development of functionally distinct fibrocartilages at two sites in the quadriceps tendon of the rat: the suprapatella and the attachment to the patella. Anat Embryol (Berl). 1992, 185, 181-187. |
| 21. | Rufai, A.; Benjamin, M.; Ralphs, J. R. Development and ageing of phenotypically distinct fibrocartilages associated with the rat Achilles tendon. Anat Embryol (Berl). 1992, 186, 611-618. |
| 22. |
Blitz, E.; Viukov, S.; Sharir, A.; Shwartz, Y.; Galloway, J. L.; Pryce, B. A.; Johnson, R. L.; Tabin, C. J.; Schweitzer, R.; Zelzer, E. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009, 17, 861-873.
doi: 10.1016/j.devcel.2009.10.010 URL |
| 23. |
Blitz, E.; Sharir, A.; Akiyama, H.; Zelzer, E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development. 2013, 140, 2680-2690.
doi: 10.1242/dev.093906 URL |
| 24. |
Galatz, L.; Rothermich, S.; VanderPloeg, K.; Petersen, B.; Sandell, L.; Thomopoulos, S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007, 25, 1621-1628.
doi: 10.1002/(ISSN)1554-527X URL |
| 25. |
Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; Asahara, H. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc Natl Acad Sci U S A. 2010, 107, 10538-10542.
doi: 10.1073/pnas.1000525107 URL |
| 26. |
Schweitzer, R.; Chyung, J. H.; Murtaugh, L. C.; Brent, A. E.; Rosen, V.; Olson, E. N.; Lassar, A.; Tabin, C. J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001, 128, 3855-3866.
doi: 10.1242/dev.128.19.3855 URL |
| 27. |
Sugimoto, Y.; Takimoto, A.; Akiyama, H.; Kist, R.; Scherer, G.; Nakamura, T.; Hiraki, Y.; Shukunami, C. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013, 140, 2280-2288.
doi: 10.1242/dev.096354 URL |
| 28. |
Killian, M. L.; Thomopoulos, S. Scleraxis is required for the development of a functional tendon enthesis. FASEB J. 2016, 30, 301-311.
doi: 10.1096/fsb2.v30.1 URL |
| 29. |
Schwartz, A. G.; Long, F.; Thomopoulos, S. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development. 2015, 142, 196-206.
doi: 10.1242/dev.112714 URL |
| 30. |
Dyment, N. A.; Breidenbach, A. P.; Schwartz, A. G.; Russell, R. P.; Aschbacher-Smith, L.; Liu, H.; Hagiwara, Y.; Jiang, R.; Thomopoulos, S.; Butler, D. L.; Rowe, D. W. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol. 2015, 405, 96-107.
doi: 10.1016/j.ydbio.2015.06.020 URL |
| 31. | Dyment, N. A.; Hagiwara, Y.; Matthews, B. G.; Li, Y.; Kalajzic, I.; Rowe, D. W. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One. 2014, 9, e96113. |
| 32. |
Jensen, P. T.; Lambertsen, K. L.; Frich, L. H. Assembly, maturation, and degradation of the supraspinatus enthesis. J Shoulder Elbow Surg. 2018, 27, 739-750.
doi: 10.1016/j.jse.2017.10.030 URL |
| 33. |
Santoni, B. G.; McGilvray, K. C.; Lyons, A. S.; Bansal, M.; Turner, A. S.; Macgillivray, J. D.; Coleman, S. H.; Puttlitz, C. M. Biomechanical analysis of an ovine rotator cuff repair via porous patch augmentation in a chronic rupture model. Am J Sports Med. 2010, 38, 679-686.
doi: 10.1177/0363546510366866 URL |
| 34. |
Mori, D.; Funakoshi, N.; Yamashita, F.; Wakabayashi, T. Effect of fatty degeneration of the infraspinatus on the efficacy of arthroscopic patch autograft procedure for large to massive rotator cuff tears. Am J Sports Med. 2015, 43, 1108-1117.
doi: 10.1177/0363546515569680 URL |
| 35. |
Hakimi, O.; Mouthuy, P. A.; Zargar, N.; Lostis, E.; Morrey, M.; Carr, A. A layered electrospun and woven surgical scaffold to enhance endogenous tendon repair. Acta Biomater. 2015, 26, 124-135.
doi: 10.1016/j.actbio.2015.08.007 URL |
| 36. |
Thomopoulos, S.; Kim, H. M.; Rothermich, S. Y.; Biederstadt, C.; Das, R.; Galatz, L. M. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007, 25, 1154-1163.
doi: 10.1002/(ISSN)1554-527X URL |
| 37. | Tatara, A. M.; Lipner, J. H.; Das, R.; Kim, H. M.; Patel, N.; Ntouvali, E.; Silva, M. J.; Thomopoulos, S. The role of muscle loading on bone (Re)modeling at the developing enthesis. PLoS One. 2014, 9, e97375. |
| 38. |
Rhee, Y. G.; Cho, N. S.; Yoo, J. H. Clinical outcome and repair integrity after rotator cuff repair in patients older than 70 years versus patients younger than 70 years. Arthroscopy. 2014, 30, 546-554.
doi: 10.1016/j.arthro.2014.02.006 URL |
| 39. |
Melis, B.; Nemoz, C.; Walch, G. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res. 2009, 95, 319-324.
doi: 10.1016/j.otsr.2009.05.001 URL |
| 40. |
Meyer, D. C.; Farshad, M.; Amacker, N. A.; Gerber, C.; Wieser, K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012, 40, 606-610.
doi: 10.1177/0363546511429778 URL |
| 41. |
Saadat, F.; Deymier, A. C.; Birman, V.; Thomopoulos, S.; Genin, G. M. The concentration of stress at the rotator cuff tendon-to-bone attachment site is conserved across species. J Mech Behav Biomed Mater. 2016, 62, 24-32.
doi: 10.1016/j.jmbbm.2016.04.025 URL |
| 42. |
Kanazawa, T.; Gotoh, M.; Ohta, K.; Honda, H.; Ohzono, H.; Shimokobe, H.; Shiba, N.; Nakamura, K. Histomorphometric and ultrastructural analysis of the tendon-bone interface after rotator cuff repair in a rat model. Sci Rep. 2016, 6, 33800.
doi: 10.1038/srep33800 |
| 43. |
Angeline, M. E.; Rodeo, S. A. Biologics in the management of rotator cuff surgery. Clin Sports Med. 2012, 31, 645-663.
doi: 10.1016/j.csm.2012.07.003 URL |
| 44. |
Harryman, D. T., 2nd; Mack, L. A.; Wang, K. Y.; Jackins, S. E.; Richardson, M. L.; Matsen, F. A., 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991, 73, 982-989.
doi: 10.2106/00004623-199173070-00004 URL |
| 45. |
Liem, D.; Lichtenberg, S.; Magosch, P.; Habermeyer, P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am. 2007, 89, 1770-1776.
doi: 10.2106/JBJS.F.00749 URL |
| 46. |
Charousset, C.; Bellaïche, L.; Kalra, K.; Petrover, D. Arthroscopic repair of full-thickness rotator cuff tears: is there tendon healing in patients aged 65 years or older? Arthroscopy. 2010, 26, 302-309.
doi: 10.1016/j.arthro.2009.08.027 URL |
| 47. |
Cho, N. S.; Rhee, Y. G. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009, 1, 96-104.
doi: 10.4055/cios.2009.1.2.96 URL |
| 48. |
Nho, S. J.; Shindle, M. K.; Adler, R. S.; Warren, R. F.; Altchek, D. W.; MacGillivray, J. D. Prospective analysis of arthroscopic rotator cuff repair: subgroup analysis. J Shoulder Elbow Surg. 2009, 18, 697-704.
doi: 10.1016/j.jse.2008.11.018 URL |
| 49. |
Oh, J. H.; Kim, S. H.; Kang, J. Y.; Oh, C. H.; Gong, H. S. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010, 38, 672-678.
doi: 10.1177/0363546509352460 URL |
| 50. |
Tashjian, R. Z.; Hollins, A. M.; Kim, H. M.; Teefey, S. A.; Middleton, W. D.; Steger-May, K.; Galatz, L. M.; Yamaguchi, K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010, 38, 2435-2442.
doi: 10.1177/0363546510382835 URL |
| 51. | Boileau, P.; Brassart, N.; Watkinson, D. J.; Carles, M.; Hatzidakis, A. M.; Krishnan, S. G. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005, 87, 1229-1240. |
| 52. | Thomazeau, H.; Boukobza, E.; Morcet, N.; Chaperon, J.; Langlais, F. Prediction of rotator cuff repair results by magnetic resonance imaging. Clin Orthop Relat Res. 1997, 275-283. |
| 53. |
Goutallier, D.; Postel, J. M.; Gleyze, P.; Leguilloux, P.; Van Driessche, S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003, 12, 550-554.
doi: 10.1016/S1058-2746(03)00211-8 URL |
| 54. | Galatz, L. M.; Silva, M. J.; Rothermich, S. Y.; Zaegel, M. A.; Havlioglu, N.; Thomopoulos, S. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am. 2006, 88, 2027-2034. |
| 55. |
Bedi, A.; Fox, A. J.; Harris, P. E.; Deng, X. H.; Ying, L.; Warren, R. F.; Rodeo, S. A. Diabetes mellitus impairs tendon-bone healing after rotator cuff repair. J Shoulder Elbow Surg. 2010, 19, 978-988.
doi: 10.1016/j.jse.2009.11.045 URL |
| 56. |
Chen, A. L.; Shapiro, J. A.; Ahn, A. K.; Zuckerman, J. D.; Cuomo, F. Rotator cuff repair in patients with type I diabetes mellitus. J Shoulder Elbow Surg. 2003, 12, 416-421.
doi: 10.1016/S1058-2746(03)00172-1 URL |
| 57. |
Tilley, B. J.; Cook, J. L.; Docking, S. I.; Gaida, J. E. Is higher serum cholesterol associated with altered tendon structure or tendon pain? A systematic review. Br J Sports Med. 2015, 49, 1504-1509.
doi: 10.1136/bjsports-2015-095100 URL |
| 58. |
Bolam, S. M.; Park, Y. E.; Konar, S.; Callon, K. E.; Workman, J.; Monk, A. P.; Coleman, B.; Cornish, J.; Vickers, M. H.; Munro, J. T.; Musson, D. S. Obesity impairs enthesis healing after rotator cuff repair in a rat model. Am J Sports Med. 2021, 49, 3959-3969.
doi: 10.1177/03635465211049219 URL |
| 59. |
Angeline, M. E.; Ma, R.; Pascual-Garrido, C.; Voigt, C.; Deng, X. H.; Warren, R. F.; Rodeo, S. A. Effect of diet-induced vitamin D deficiency on rotator cuff healing in a rat model. Am J Sports Med. 2014, 42, 27-34.
doi: 10.1177/0363546513505421 URL |
| 60. |
Ryu, K. J.; Kim, B. H.; Lee, Y.; Dan, J.; Kim, J. H. Low serum vitamin d is not correlated with the severity of a rotator cuff tear or retear after arthroscopic repair. Am J Sports Med. 2015, 43, 1743-1750.
doi: 10.1177/0363546515578101 URL |
| 61. |
Chung, S. W.; Oh, J. H.; Gong, H. S.; Kim, J. Y.; Kim, S. H. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011, 39, 2099-2107.
doi: 10.1177/0363546511415659 URL |
| 62. |
Cohen, D. B.; Kawamura, S.; Ehteshami, J. R.; Rodeo, S. A. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006, 34, 362-369.
doi: 10.1177/0363546505280428 URL |
| 63. | Chan, K. M.; Fu, S. C. Anti-inflammatory management for tendon injuries - friends or foes? Sports Med Arthrosc Rehabil Ther Technol. 2009, 1, 23. |
| 64. |
Rak Kwon, D.; Jung, S.; Jang, J.; Park, G. Y.; Suk Moon, Y.; Lee, S. C. A 3-Dimensional bioprinted scaffold with human umbilical cord blood-mesenchymal stem cells improves regeneration of chronic full-thickness rotator cuff tear in a rabbit model. Am J Sports Med. 2020, 48, 947-958.
doi: 10.1177/0363546520904022 URL |
| 65. |
García-Gómez, I.; Elvira, G.; Zapata, A. G.; Lamana, M. L.; Ramírez, M.; Castro, J. G.; Arranz, M. G.; Vicente, A.; Bueren, J.; García-Olmo, D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010, 10, 1453-1468.
doi: 10.1517/14712598.2010.519333 URL |
| 66. | Lee, E. H.; Hui, J. H. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg Br. 2006, 88, 841-851. |
| 67. |
Morton-Gonzaba, N.; Carlisle, D.; Emukah, C.; Chorath, K.; Moreira, A. Mesenchymal stem cells and their application to rotator cuff pathology: A meta-analysis of pre-clinical studies. Osteoarthr Cartil Open. 2020, 2, 100047.
doi: 10.1016/j.ocarto.2020.100047 URL |
| 68. |
Honda, H.; Gotoh, M.; Kanazawa, T.; Ohzono, H.; Nakamura, H.; Ohta, K.; Nakamura, K. I.; Fukuda, K.; Teramura, T.; Hashimoto, T.; Shichijo, S.; Shiba, N. Hyaluronic acid accelerates tendon-to-bone healing after rotator cuff repair. Am J Sports Med. 2017, 45, 3322-3330.
doi: 10.1177/0363546517720199 URL |
| 69. |
Jo, C. H.; Shin, J. S.; Park, I. W.; Kim, H.; Lee, S. Y. Multiple channeling improves the structural integrity of rotator cuff repair. Am J Sports Med. 2013, 41, 2650-2657.
doi: 10.1177/0363546513499138 URL |
| 70. |
Hernigou, P.; Flouzat Lachaniette, C. H.; Delambre, J.; Zilber, S.; Duffiet, P.; Chevallier, N.; Rouard, H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014, 38, 1811-1818.
doi: 10.1007/s00264-014-2391-1 URL |
| 71. |
Taniguchi, N.; Suenaga, N.; Oizumi, N.; Miyoshi, N.; Yamaguchi, H.; Inoue, K.; Chosa, E. Bone marrow stimulation at the footprint of arthroscopic surface-holding repair advances cuff repair integrity. J Shoulder Elbow Surg. 2015, 24, 860-866.
doi: 10.1016/j.jse.2014.09.031 URL |
| 72. | Han, L.; Fang, W. L.; Jin, B.; Xu, S. C.; Zheng, X.; Hu, Y. G. Enhancement of tendon-bone healing after rotator cuff injuries using combined therapy with mesenchymal stem cells and platelet rich plasma. Eur Rev Med Pharmacol Sci. 2019, 23, 9075-9084. |
| 73. |
Gulotta, L. V.; Kovacevic, D.; Ehteshami, J. R.; Dagher, E.; Packer, J. D.; Rodeo, S. A. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009, 37, 2126-2133.
doi: 10.1177/0363546509339582 URL |
| 74. | Nourissat, G.; Diop, A.; Maurel, N.; Salvat, C.; Dumont, S.; Pigenet, A.; Gosset, M.; Houard, X.; Berenbaum, F. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS One. 2010, 5, e12248. |
| 75. |
Gulotta, L. V.; Kovacevic, D.; Montgomery, S.; Ehteshami, J. R.; Packer, J. D.; Rodeo, S. A. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med. 2010, 38, 1429-1437.
doi: 10.1177/0363546510361235 URL |
| 76. |
Vishnubalaji, R.; Al-Nbaheen, M.; Kadalmani, B.; Aldahmash, A.; Ramesh, T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012, 347, 419-427.
doi: 10.1007/s00441-011-1306-3 URL |
| 77. |
Oh, J. H.; Chung, S. W.; Kim, S. H.; Chung, J. Y.; Kim, J. Y. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg. 2014, 23, 445-455.
doi: 10.1016/j.jse.2013.07.054 URL |
| 78. | Chen, Y.; Xu, Y.; Dai, G.; Shi, Q.; Duan, C. Enhanced Repaired Enthesis Using Tenogenically Differentiated Adipose-Derived Stem Cells in a Murine Rotator Cuff Injury Model. Stem Cells Int. 2022, 2022, 1309684. |
| 79. | Choi, J. H.; Shim, I. K.; Shin, M. J.; Lee, Y. N.; Koh, K. H. Stem cell sheet interpositioned between the tendon and bone would be better for healing than stem cell sheet overlaid above the tendon-to-bone junction in rotator cuff repair of rats. PLoS One. 2022, 17, e0266030. |
| 80. | Valencia Mora, M.; Antuña Antuña, S.; García Arranz, M.; Carrascal, M. T.; Barco, R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014, 45 Suppl 4, S22-27. |
| 81. |
Rothrauff, B. B.; Smith, C. A.; Ferrer, G. A.; Novaretti, J. V.; Pauyo, T.; Chao, T.; Hirsch, D.; Beaudry, M. F.; Herbst, E.; Tuan, R. S.; Debski, R. E.; Musahl, V. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J Shoulder Elbow Surg. 2019, 28, 654-664.
doi: 10.1016/j.jse.2018.08.044 URL |
| 82. |
Zong, J. C.; Mosca, M. J.; Degen, R. M.; Lebaschi, A.; Carballo, C.; Carbone, A.; Cong, G. T.; Ying, L.; Deng, X. H.; Rodeo, S. A. Involvement of Indian hedgehog signaling in mesenchymal stem cell-augmented rotator cuff tendon repair in an athymic rat model. J Shoulder Elbow Surg. 2017, 26, 580-588.
doi: 10.1016/j.jse.2016.09.036 URL |
| 83. | Schwartz, A. G.; Galatz, L. M.; Thomopoulos, S. Enthesis regeneration: a role for Gli1+ progenitor cells. Development. 2017, 144, 1159-1164. |
| 84. |
Nakazawa, T.; Nakajima, A.; Shiomi, K.; Moriya, H.; Einhorn, T. A.; Yamazaki, M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005, 37, 711-719.
doi: 10.1016/j.bone.2005.06.013 URL |
| 85. |
Kakar, S.; Einhorn, T. A.; Vora, S.; Miara, L. J.; Hon, G.; Wigner, N. A.; Toben, D.; Jacobsen, K. A.; Al-Sebaei, M. O.; Song, M.; Trackman, P. C.; Morgan, E. F.; Gerstenfeld, L. C.; Barnes, G. L. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007, 22, 1903-1912.
doi: 10.1359/jbmr.070724 URL |
| 86. | Chen, Y.; Bai, B.; Zhang, S.; Ye, J.; Chen, Y.; Zeng, Y. Effects of parathyroid hormone on calcium ions in rat bone marrow mesenchymal stem cells. Biomed Res Int. 2014, 2014, 258409. |
| 87. |
Hettrich, C. M.; Beamer, B. S.; Bedi, A.; Deland, K.; Deng, X. H.; Ying, L.; Lane, J.; Rodeo, S. A. The effect of rhPTH on the healing of tendon to bone in a rat model. J Orthop Res. 2012, 30, 769-774.
doi: 10.1002/jor.v30.5 URL |
| 88. |
Duchman, K. R.; Goetz, J. E.; Uribe, B. U.; Amendola, A. M.; Barber, J. A.; Malandra, A. E.; Fredericks, D. C.; Hettrich, C. M. Delayed administration of recombinant human parathyroid hormone improves early biomechanical strength in a rat rotator cuff repair model. J Shoulder Elbow Surg. 2016, 25, 1280-1287.
doi: 10.1016/j.jse.2015.12.016 URL |
| 89. |
Oh, J. H.; Kim, D. H.; Jeong, H. J.; Park, J. H.; Rhee, S. M. Effect of recombinant human parathyroid hormone on rotator cuff healing after arthroscopic repair. Arthroscopy. 2019, 35, 1064-1071.
doi: 10.1016/j.arthro.2018.11.038 URL |
| 90. |
Gross, G.; Hoffmann, A. Therapeutic strategies for tendon healing based on novel biomaterials, factors and cells. Pathobiology. 2013, 80, 203-210.
doi: 10.1159/000347059 URL |
| 91. |
Caliari, S. R.; Harley, B. A. Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen-GAG scaffolds. Tissue Eng Part A. 2013, 19, 1100-1112.
doi: 10.1089/ten.tea.2012.0497 URL |
| 92. |
Hee, C. K.; Dines, J. S.; Dines, D. M.; Roden, C. M.; Wisner-Lynch, L. A.; Turner, A. S.; McGilvray, K. C.; Lyons, A. S.; Puttlitz, C. M.; Santoni, B. G. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am J Sports Med. 2011, 39, 1630-1639.
doi: 10.1177/0363546511404942 URL |
| 93. | Novakova, S. S.; Mahalingam, V. D.; Florida, S. E.; Mendias, C. L.; Allen, A.; Arruda, E. M.; Bedi, A.; Larkin, L. M. Tissue-engineered tendon constructs for rotator cuff repair in sheep. J Orthop Res. 2018, 36, 289-299. |
| 94. |
Tokunaga, T.; Shukunami, C.; Okamoto, N.; Taniwaki, T.; Oka, K.; Sakamoto, H.; Ide, J.; Mizuta, H.; Hiraki, Y. FGF-2 stimulates the growth of tenogenic progenitor cells to facilitate the generation of tenomodulin-positive tenocytes in a rat rotator cuff healing model. Am J Sports Med. 2015, 43, 2411-2422.
doi: 10.1177/0363546515597488 URL |
| 95. |
Uggen, C.; Dines, J.; McGarry, M.; Grande, D.; Lee, T.; Limpisvasti, O. The effect of recombinant human platelet-derived growth factor BB-coated sutures on rotator cuff healing in a sheep model. Arthroscopy. 2010, 26, 1456-1462.
doi: 10.1016/j.arthro.2010.02.025 URL |
| 96. | Zhao, S.; Zhao, J.; Dong, S.; Huangfu, X.; Li, B.; Yang, H.; Zhao, J.; Cui, W. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int J Nanomedicine. 2014, 9, 2373-2385. |
| 97. |
Sahoo, S.; Toh, S. L.; Goh, J. C. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010, 31, 2990-2998.
doi: 10.1016/j.biomaterials.2010.01.004 URL |
| 98. |
Ker, E. D.; Nain, A. S.; Weiss, L. E.; Wang, J.; Suhan, J.; Amon, C. H.; Campbell, P. G. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials. 2011, 32, 8097-8107.
doi: 10.1016/j.biomaterials.2011.07.025 URL |
| 99. |
James, R.; Kumbar, S. G.; Laurencin, C. T.; Balian, G.; Chhabra, A. B. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011, 6, 025011.
doi: 10.1088/1748-6041/6/2/025011 URL |
| 100. |
Raabe, O.; Shell, K.; Fietz, D.; Freitag, C.; Ohrndorf, A.; Christ, H. J.; Wenisch, S.; Arnhold, S. Tenogenic differentiation of equine adipose-tissue-derived stem cells under the influence of tensile strain, growth differentiation factors and various oxygen tensions. Cell Tissue Res. 2013, 352, 509-521.
doi: 10.1007/s00441-013-1574-1 URL |
| 101. |
Holladay, C.; Abbah, S. A.; O'Dowd, C.; Pandit, A.; Zeugolis, D. I. Preferential tendon stem cell response to growth factor supplementation. J Tissue Eng Regen Med. 2016, 10, 783-798.
doi: 10.1002/term.v10.9 URL |
| 102. |
Durant, T. J.; Dyment, N.; McCarthy, M. B.; Cote, M. P.; Arciero, R. A.; Mazzocca, A. D.; Rowe, D. Mesenchymal stem cell response to growth factor treatment and low oxygen tension in 3-dimensional construct environment. Muscles Ligaments Tendons J. 2014, 4, 46-51.
doi: 10.32098/mltj.01.2014.09 URL |
| 103. |
Cheng, X.; Tsao, C.; Sylvia, V. L.; Cornet, D.; Nicolella, D. P.; Bredbenner, T. L.; Christy, R. J. Platelet-derived growth-factor-releasing aligned collagen-nanoparticle fibers promote the proliferation and tenogenic differentiation of adipose-derived stem cells. Acta Biomater. 2014, 10, 1360-1369.
doi: 10.1016/j.actbio.2013.11.017 URL |
| 104. |
Manning, C. N.; Schwartz, A. G.; Liu, W.; Xie, J.; Havlioglu, N.; Sakiyama-Elbert, S. E.; Silva, M. J.; Xia, Y.; Gelberman, R. H.; Thomopoulos, S. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater. 2013, 9, 6905-6914.
doi: 10.1016/j.actbio.2013.02.008 URL |
| 105. |
Würgler-Hauri, C. C.; Dourte, L. M.; Baradet, T. C.; Williams, G. R.; Soslowsky, L. J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007, 16, S198-203.
doi: 10.1016/j.jse.2007.04.003 URL |
| 106. |
Kobayashi, M.; Itoi, E.; Minagawa, H.; Miyakoshi, N.; Takahashi, S.; Tuoheti, Y.; Okada, K.; Shimada, Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006, 15, 371-377.
doi: 10.1016/j.jse.2005.09.003 URL |
| 107. |
Rodeo, S. A.; Potter, H. G.; Kawamura, S.; Turner, A. S.; Kim, H. J.; Atkinson, B. L. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007, 89, 2485-2497.
doi: 10.2106/00004623-200711000-00021 URL |
| 108. |
Kim, H. M.; Galatz, L. M.; Das, R.; Havlioglu, N.; Rothermich, S. Y.; Thomopoulos, S. The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect Tissue Res. 2011, 52, 87-98.
doi: 10.3109/03008207.2010.483026 URL |
| 109. |
Kovacevic, D.; Fox, A. J.; Bedi, A.; Ying, L.; Deng, X. H.; Warren, R. F.; Rodeo, S. A. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med. 2011, 39, 811-819.
doi: 10.1177/0363546511399378 URL |
| 110. |
Manning, C. N.; Kim, H. M.; Sakiyama-Elbert, S.; Galatz, L. M.; Havlioglu, N.; Thomopoulos, S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res. 2011, 29, 1099-1105.
doi: 10.1002/jor.21301 URL |
| 111. |
Nagura, I.; Kokubu, T.; Mifune, Y.; Inui, A.; Takase, F.; Ueda, Y.; Kataoka, T.; Kurosaka, M. Characterization of progenitor cells derived from torn human rotator cuff tendons by gene expression patterns of chondrogenesis, osteogenesis, and adipogenesis. J Orthop Surg Res. 2016, 11, 40.
doi: 10.1186/s13018-016-0373-2 URL |
| 112. | Davies, M. R.; Liu, X.; Lee, L.; Laron, D.; Ning, A. Y.; Kim, H. T.; Feeley, B. T. TGF-β small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One. 2016, 11, e0155486. |
| 113. |
Zhou, Z.; Song, W.; Zhang, G.; Zhan, S.; Cai, Z.; Yu, W.; He, Y. The recombinant human fibroblast growth factor-18 (sprifermin) improves tendon-to-bone healing by promoting chondrogenesis in a rat rotator cuff repair model. J Shoulder Elbow Surg. 2022, 31, 1617-1627.
doi: 10.1016/j.jse.2022.01.137 URL |
| 114. |
Sitcheran, R.; Cogswell, P. C.; Baldwin, A. S., Jr. NF-kappaB mediates inhibition of mesenchymal cell differentiation through a posttranscriptional gene silencing mechanism. Genes Dev. 2003, 17, 2368-2373.
doi: 10.1101/gad.1114503 URL |
| 115. |
Gulotta, L. V.; Kovacevic, D.; Cordasco, F.; Rodeo, S. A. Evaluation of tumor necrosis factor α blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy. 2011, 27, 1351-1357.
doi: 10.1016/j.arthro.2011.03.076 URL |
| 116. |
Waldorff, E. I.; Lindner, J.; Kijek, T. G.; Downie, B. K.; Hughes, R. E.; Carpenter, J. E.; Miller, B. S. Bone density of the greater tuberosity is decreased in rotator cuff disease with and without full-thickness tears. J Shoulder Elbow Surg. 2011, 20, 904-908.
doi: 10.1016/j.jse.2010.12.009 URL |
| 117. | Dorman, L. J.; Tucci, M.; Benghuzzi, H. In vitro effects of bmp-2, bmp-7, and bmp-13 on proliferation and differentation of mouse mesenchymal stem cells. Biomed Sci Instrum. 2012, 48, 81-87. |
| 118. |
Kabuto, Y.; Morihara, T.; Sukenari, T.; Kida, Y.; Oda, R.; Arai, Y.; Sawada, K.; Matsuda, K.; Kawata, M.; Tabata, Y.; Fujiwara, H.; Kubo, T. Stimulation of rotator cuff repair by sustained release of bone morphogenetic protein-7 using a gelatin hydrogel sheet. Tissue Eng Part A. 2015, 21, 2025-2033.
doi: 10.1089/ten.tea.2014.0541 URL |
| 119. |
Dahlgren, L. A.; Mohammed, H. O.; Nixon, A. J. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005, 23, 84-92.
doi: 10.1016/j.orthres.2004.05.007 URL |
| 120. |
Carr, J. B., 2nd Rodeo, S. A. The role of biologic agents in the management of common shoulder pathologies: current state and future directions. J Shoulder Elbow Surg. 2019, 28, 2041-2052.
doi: 10.1016/j.jse.2019.07.025 URL |
| 121. |
Neviaser, J. S.; Neviaser, R. J.; Neviaser, T. J. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978, 60, 681-684.
doi: 10.2106/00004623-197860050-00017 URL |
| 122. |
Dejardin, L. M.; Arnoczky, S. P.; Ewers, B. J.; Haut, R. C.; Clarke, R. B. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001, 29, 175-184.
doi: 10.1177/03635465010290021001 URL |
| 123. | Zheng, M. H.; Chen, J.; Kirilak, Y.; Willers, C.; Xu, J.; Wood, D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005, 73, 61-67. |
| 124. |
Huang, C.; Zhang, X.; Luo, H.; Pan, J.; Cui, W.; Cheng, B.; Zhao, S.; Chen, G. Effect of kartogenin-loaded gelatin methacryloyl hydrogel scaffold with bone marrow stimulation for enthesis healing in rotator cuff repair. J Shoulder Elbow Surg. 2021, 30, 544-553.
doi: 10.1016/j.jse.2020.06.013 URL |
| 125. |
Han, L.; Liu, H.; Fu, H.; Hu, Y.; Fang, W.; Liu, J. Exosome-delivered BMP-2 and polyaspartic acid promotes tendon bone healing in rotator cuff tear via Smad/RUNX2 signaling pathway. Bioengineered. 2022, 13, 1459-1475.
doi: 10.1080/21655979.2021.2019871 URL |
| 126. |
Ousema, P. H.; Moutos, F. T.; Estes, B. T.; Caplan, A. I.; Lennon, D. P.; Guilak, F.; Weinberg, J. B. The inhibition by interleukin 1 of MSC chondrogenesis and the development of biomechanical properties in biomimetic 3D woven PCL scaffolds. Biomaterials. 2012, 33, 8967-8974.
doi: 10.1016/j.biomaterials.2012.08.045 URL |
| 127. | Jiang, X.; Wu, S.; Kuss, M.; Kong, Y.; Shi, W.; Streubel, P. N.; Li, T.; Duan, B. 3D printing of multilayered scaffolds for rotator cuff tendon regeneration. Bioact Mater. 2020, 5, 636-643. |
| 128. |
Ciampi, P.; Scotti, C.; Nonis, A.; Vitali, M.; Di Serio, C.; Peretti, G. M.; Fraschini, G. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014, 42, 1169-1175.
doi: 10.1177/0363546514525592 URL |
| 129. |
Cai, Y. Z.; Zhang, C.; Jin, R. L.; Shen, T.; Gu, P. C.; Lin, X. J.; Chen, J. D. Arthroscopic rotator cuff repair with graft augmentation of 3-dimensional biological collagen for moderate to large tears: a randomized controlled study. Am J Sports Med. 2018, 46, 1424-1431.
doi: 10.1177/0363546518756978 URL |
| 130. |
Sclamberg, S. G.; Tibone, J. E.; Itamura, J. M.; Kasraeian, S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg. 2004, 13, 538-541.
doi: 10.1016/j.jse.2004.03.005 URL |
| 131. |
Iannotti, J. P.; Codsi, M. J.; Kwon, Y. W.; Derwin, K.; Ciccone, J.; Brems, J. J. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006, 88, 1238-1244.
doi: 10.2106/JBJS.E.00524 URL |
| 132. | Malcarney, H. L.; Bonar, F.; Murrell, G. A. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant:a report of 4 cases. Am J Sports Med. 2005, 33, 907-911. |
| 133. |
Hoberman, A. R.; Cirino, C.; McCarthy, M. B.; Cote, M. P.; Pauzenberger, L.; Beitzel, K.; Mazzocca, A. D.; Dyrna, F. Bone marrow-derived mesenchymal stromal cells enhanced by platelet-rich plasma maintain adhesion to scaffolds in arthroscopic simulation. Arthroscopy. 2018, 34, 872-881.
doi: 10.1016/j.arthro.2017.08.291 URL |
| 134. |
Thangarajah, T.; Henshaw, F.; Sanghani-Kerai, A.; Lambert, S. M.; Blunn, G. W.; Pendegrass, C. J. The effectiveness of demineralized cortical bone matrix in a chronic rotator cuff tear model. J Shoulder Elbow Surg. 2017, 26, 619-626.
doi: 10.1016/j.jse.2017.01.003 URL |
| 135. |
Thangarajah, T.; Sanghani-Kerai, A.; Henshaw, F.; Lambert, S. M.; Pendegrass, C. J.; Blunn, G. W. Application of a demineralized cortical bone matrix and bone marrow-derived mesenchymal stem cells in a model of chronic rotator cuff degeneration. Am J Sports Med. 2018, 46, 98-108.
doi: 10.1177/0363546517727512 URL |
| 136. | Smith, M. J.; Pfeiffer, F. M.; Cook, C. R.; Kuroki, K.; Cook, J. L. Rotator cuff healing using demineralized cancellous bone matrix sponge interposition compared to standard repair in a preclinical canine model. J Orthop Res. 2018, 36, 906-912. |
| 137. |
Wellington, I. J.; Muench, L. N.; Hawthorne, B. C.; Uyeki, C. L.; Antonacci, C. L.; McCarthy, M. B.; Connors, J. P.; Kia, C.; Mazzocca, A. D.; Berthold, D. P. Clinical outcomes following biologically enhanced demineralized bone matrix augmentation of complex rotator cuff repair. J Clin Med. 2022, 11, 2956.
doi: 10.3390/jcm11112956 URL |
| 138. | Chae, S.; Yong, U.; Park, W.; Choi, Y. M.; Jeon, I. H.; Kang, H.; Jang, J.; Choi, H. S.; Cho, D. W. 3D cell-printing of gradient multi-tissue interfaces for rotator cuff regeneration. Bioact Mater. 2023, 19, 611-625. |
| 139. | Yoon, J. Y.; Park, J. H.; Rhee, S. M.; Jeong, H. J.; Han, J.; Lee, J. H.; Jeon, S.; Oh, J. H. Safety and efficacy of autologous dermal fibroblast injection to enhance healing after full-thickness rotator cuff repair: first-in-human pilot study. Orthop J Sports Med. 2021, 9, 23259671211052996. |
| 140. | Randelli, P.; Randelli, F.; Ragone, V.; Menon, A.; D'Ambrosi, R.; Cucchi, D.; Cabitza, P.; Banfi, G. Regenerative medicine in rotator cuff injuries. Biomed Res Int. 2014, 2014, 129515. |
| 141. |
Warth, R. J.; Dornan, G. J.; James, E. W.; Horan, M. P.; Millett, P. J. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015, 31, 306-320.
doi: 10.1016/j.arthro.2014.09.007 URL |
| 142. |
Castricini, R.; Longo, U. G.; De Benedetto, M.; Panfoli, N.; Pirani, P.; Zini, R.; Maffulli, N.; Denaro, V. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011, 39, 258-265.
doi: 10.1177/0363546510390780 URL |
| 143. |
Mazzocca, A. D.; McCarthy, M. B.; Chowaniec, D. M.; Cote, M. P.; Romeo, A. A.; Bradley, J. P.; Arciero, R. A.; Beitzel, K. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012, 94, 308-316.
doi: 10.2106/JBJS.K.00430 URL |
| 144. |
Giovannetti de Sanctis, E.; Franceschetti, E.; De Dona, F.; Palumbo, A.; Paciotti, M.; Franceschi, F. The efficacy of injections for partial rotator cuff tears: a systematic review. J Clin Med. 2020, 10, 51.
doi: 10.3390/jcm10010051 URL |
| 145. |
von Wehren, L.; Blanke, F.; Todorov, A.; Heisterbach, P.; Sailer, J.; Majewski, M. The effect of subacromial injections of autologous conditioned plasma versus cortisone for the treatment of symptomatic partial rotator cuff tears. Knee Surg Sports Traumatol Arthrosc. 2016, 24, 3787-3792.
doi: 10.1007/s00167-015-3651-3 URL |
| 146. | Xu, W.; Xue, Q. Application of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review and meta-analysis. Orthop J Sports Med. 2021, 9, 23259671211016847. |
| 147. |
Rha, D. W.; Park, G. Y.; Kim, Y. K.; Kim, M. T.; Lee, S. C. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013, 27, 113-122.
doi: 10.1177/0269215512448388 URL |
| 148. |
Shams, A.; El-Sayed, M.; Gamal, O.; Ewes, W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016, 26, 837-842.
doi: 10.1007/s00590-016-1826-3 URL |
| 149. | Cai, Y. U.; Sun, Z.; Liao, B.; Song, Z.; Xiao, T.; Zhu, P. Sodium hyaluronate and platelet-rich plasma for partial-thickness rotator cuff tears. Med Sci Sports Exerc. 2019, 51, 227-233. |
| 150. |
Ryan, J.; Imbergamo, C.; Sudah, S.; Kirchner, G.; Greenberg, P.; Monica, J.; Gatt, C. Platelet-rich product supplementation in rotator cuff repair reduces retear rates and improves clinical outcomes: a meta-analysis of randomized controlled trials. Arthroscopy. 2021, 37, 2608-2624.
doi: 10.1016/j.arthro.2021.03.010 URL |
| 151. |
Lavoie-Gagne, O.; Fury, M. S.; Mehta, N.; Harkin, W. E.; Bernstein, D. N.; Berlinberg, E. J.; Parvaresh, K.; O'Donnell, E.; Forsythe, B. Double-row repair with platelet-rich plasma optimizes retear rates after small to medium full-thickness rotator cuff repair: a systematic review and network meta-analysis of randomized controlled trials. Arthroscopy. 2022, 38, 2714-2729.
doi: 10.1016/j.arthro.2022.03.014 URL |
| 152. | Ilhanli, I.; Guder, N.; Gul, M. Platelet-rich plasma treatment with physical therapy in chronic partial supraspinatus tears. Iran Red Crescent Med J. 2015, 17, e23732. |
| 153. |
Anz, A. W.; Parsa, R. S.; Romero-Creel, M. F.; Nabors, A.; Tucker, M. S.; Harrison, R. M.; Matuska, A. M. Exercise-mobilized platelet-rich plasma: short-term exercise increases stem cell and platelet concentrations in platelet-rich plasma. Arthroscopy. 2019, 35, 192-200.
doi: 10.1016/j.arthro.2018.06.043 URL |
| 154. |
Osborne, J. D.; Gowda, A. L.; Wiater, B.; Wiater, J. M. Rotator cuff rehabilitation: current theories and practice. Phys Sportsmed. 2016, 44, 85-92.
doi: 10.1080/00913847.2016.1108883 URL |
| 155. | Xiao, H.; Zhang, T.; Li, C.; Cao, Y.; Wang, L.; Chen, H.; Li, S.; Guan, C.; Hu, J.; Chen, D.; Chen, C.; Lu, H. Mechanical stimulation promotes enthesis injury repair by mobilizing Prrx1(+) cells via ciliary TGF-β signaling. Elife. 2022, 11, e73614. |
| 156. |
Galatz, L. M.; Charlton, N.; Das, R.; Kim, H. M.; Havlioglu, N.; Thomopoulos, S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg. 2009, 18, 669-675.
doi: 10.1016/j.jse.2009.02.016 URL |
| 157. |
Gimbel, J. A.; Van Kleunen, J. P.; Williams, G. R.; Thomopoulos, S.; Soslowsky, L. J. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007, 129, 400-404.
doi: 10.1115/1.2721075 URL |
| 158. |
Hettrich, C. M.; Gasinu, S.; Beamer, B. S.; Stasiak, M.; Fox, A.; Birmingham, P.; Ying, O.; Deng, X. H.; Rodeo, S. A. The effect of mechanical load on tendon-to-bone healing in a rat model. Am J Sports Med. 2014, 42, 1233-1241.
doi: 10.1177/0363546514526138 URL |
| 159. |
Chen, H.; Li, S.; Xiao, H.; Wu, B.; Zhou, L.; Hu, J.; Lu, H. Effect of exercise intensity on the healing of the bone-tendon interface: a mouse rotator cuff injury model study. Am J Sports Med. 2021, 49, 2064-2073.
doi: 10.1177/03635465211011751 URL |
| 160. |
Teunis, T.; Lubberts, B.; Reilly, B. T.; Ring, D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J Shoulder Elbow Surg. 2014, 23, 1913-1921.
doi: 10.1016/j.jse.2014.08.001 URL |
| 161. |
Kaplan, L. D.; Flanigan, D. C.; Norwig, J.; Jost, P.; Bradley, J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005, 33, 1142-1146.
doi: 10.1177/0363546505274718 URL |
| 162. |
Mall, N. A.; Lee, A. S.; Chahal, J.; Sherman, S. L.; Romeo, A. A.; Verma, N. N.; Cole, B. J. An evidenced-based examination of the epidemiology and outcomes of traumatic rotator cuff tears. Arthroscopy. 2013, 29, 366-376.
doi: 10.1016/j.arthro.2012.06.024 URL |
| 163. |
Altintas, B.; Anderson, N.; Dornan, G. J.; Boykin, R. E.; Logan, C.; Millett, P. J. Return to sport after arthroscopic rotator cuff repair: is there a difference between the recreational and the competitive athlete? Am J Sports Med. 2020, 48, 252-261.
doi: 10.1177/0363546519825624 URL |
| 164. |
Burns, J. P.; Snyder, S. J. Arthroscopic rotator cuff repair in patients younger than fifty years of age. J Shoulder Elbow Surg. 2008, 17, 90-96.
doi: 10.1016/j.jse.2007.05.006 URL |
| 165. |
Antoni, M.; Klouche, S.; Mas, V.; Ferrand, M.; Bauer, T.; Hardy, P. Return to recreational sport and clinical outcomes with at least 2years follow-up after arthroscopic repair of rotator cuff tears. Orthop Traumatol Surg Res. 2016, 102, 563-567.
doi: 10.1016/j.otsr.2016.02.015 URL |
| 166. | Cao, Y. S.; Wan, Y. F. Progress on tendon-to-bone healing after rotator cuff repair. Zhongguo Gu Shang. 2018, 31, 1172-1179. |
| 167. | Font Tellado, S.; Balmayor, E. R.; Van Griensven, M. Strategies to engineer tendon/ligament-to-bone interface: Biomaterials, cells and growth factors. Adv Drug Deliv Rev. 2015, 94, 126-140. |
| 168. | Prabhath, A.; Vernekar, V. N.; Sanchez, E.; Laurencin, C. T. Growth factor delivery strategies for rotator cuff repair and regeneration. Int J Pharm. 2018, 544, 358-371. |
| 169. | Chowdhury, F.; Na, S.; Li, D.; Poh, Y. C.; Tanaka, T. S.; Wang, F.; Wang, N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010, 9, 82-88. |
| 170. | McMurray, R. J.; Gadegaard, N.; Tsimbouri, P. M.; Burgess, K. V.; McNamara, L. E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R. O.; Dalby, M. J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011, 10, 637-644. |
| 171. | Dalby, M. J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M. O.; Herzyk, P.; Wilkinson, C. D.; Oreffo, R. O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007, 6, 997-1003. |
| 172. | Gentleman, E.; Swain, R. J.; Evans, N. D.; Boonrungsiman, S.; Jell, G.; Ball, M. D.; Shean, T. A.; Oyen, M. L.; Porter, A.; Stevens, M. M. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat Mater. 2009, 8, 763-770. |
| 173. | Derwin, K. A.; Baker, A. R.; Iannotti, J. P.; McCarron, J. A. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev. 2010, 16, 21-30. |
| 174. | Ficklscherer, A.; Serr, M.; Loitsch, T.; Niethammer, T. R.; Lahner, M.; Pietschmann, M. F.; Müller, P. E. The influence of different footprint preparation techniques on tissue regeneration in rotator cuff repair in an animal model. Arch Med Sci. 2017, 13, 481-488. |
| 175. | Li, X.; Shen, P.; Su, W.; Zhao, S.; Zhao, J. Into-tunnel repair versus onto-surface repair for rotator cuff tears in a rabbit model. Am J Sports Med. 2018, 46, 1711-1719. |
| 176. | Bilsel, K.; Yildiz, F.; Kapicioglu, M.; Uzer, G.; Elmadag, M.; Pulatkan, A.; Esrefoglu, M.; Bozdag, E.; Milano, G. Efficacy of bone marrow-stimulating technique in rotator cuff repair. J Shoulder Elbow Surg. 2017, 26, 1360-1366. |
| [1] | Ross H. McWilliam, Wenlong Chang, Zhao Liu, Jiayuan Wang, Fengxuan Han, Richard A. Black, Junxi Wu, Xichun Luo, Bin Li, Wenmiao Shu. Three-dimensional biofabrication of nanosecond laser micromachined nanofibre meshes for tissue engineered scaffolds [J]. Biomaterials Translational, 2023, 4(2): 104-114. |
| [2] | Rob Jess, Tao Ling, Yi Xiong, Chris J. Wright, Feihu Zhao. Mechanical environment for in vitro cartilage tissue engineering assisted by in silico models [J]. Biomaterials Translational, 2023, 4(1): 18-26. |
| [3] | Guixin Yuan, Zan Li, Xixi Lin, Na Li, Ren Xu. New perspective of skeletal stem cells [J]. Biomaterials Translational, 2022, 3(4): 280-294. |
| [4] | Zhao–Lin Zeng, Hui Xie. Mesenchymal stem cell–derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review [J]. Biomaterials Translational, 2022, 3(3): 175-187. |
| [5] | Chavee Laomeephol, Helena Ferreira, Sorada Kanokpanont, Jittima Amie Luckanagul, Nuno M Neves, Siriporn Damrongsakkul. Osteogenic differentiation of encapsulated cells in dexamethasone–loaded phospholipid–induced silk fibroin hydrogels [J]. Biomaterials Translational, 2022, 3(3): 213-220. |
| [6] | Ricardo Donate, Maryam Tamaddon, Viviana Ribeiro, Mario Monzón, J. Miguel Oliveira, Chaozong Liu. Translation through collaboration: practice applied in BAMOS project in in vivo testing of innovative osteochondral scaffolds [J]. Biomaterials Translational, 2022, 3(2): 102-104. |
| [7] | Melika Sahranavard, Soulmaz Sarkari, SeyedehMina Safavi, Farnaz Ghorbani. Three-dimensional bio-printing of decellularized extracellular matrix-based bio-inks for cartilage regeneration: a systematic review [J]. Biomaterials Translational, 2022, 3(2): 105-115. |
| [8] | Xuechen Zhang, Ana Justo Caetano, Paul T. Sharpe, Ana Angelova Volponi. Oral stem cells, decoding and mapping the resident cells populations [J]. Biomaterials Translational, 2022, 3(1): 24-30. |
| [9] | Deepika Arora, Pamela Gehron Robey. Recent updates on the biological basis of heterogeneity in bone marrow stromal cells/skeletal stem cells [J]. Biomaterials Translational, 2022, 3(1): 3-16. |
| [10] | Suzanne M. Watt. The long and winding road: homeostatic and disordered haematopoietic microenvironmental niches: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 31-54. |
| [11] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [12] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [13] | Trivia P. Frazier, Katie Hamel, Xiying Wu, Emma Rogers, Haley Lassiter, Jordan Robinson, Omair Mohiuddin, Michael Henderson, Jeffrey M. Gimble. Adipose-derived cells: building blocks of three-dimensional microphysiological systems [J]. Biomaterials Translational, 2021, 2(4): 301-306. |
| [14] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [15] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||