Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (4): 213-233.doi: 10.12336/biomatertransl.2023.04.003
• REVIEW • Previous Articles Next Articles
Yunke Jiao1, Miao Lei1, Jianwei Zhu1, Ronghang Chang1, Xue Qu1,2,3,*( )
)
Received:2023-09-30
Revised:2023-11-13
Accepted:2023-11-24
Online:2023-12-27
Published:2023-12-28
Contact:
Xue Qu, quxue@ecust.edu.cn.
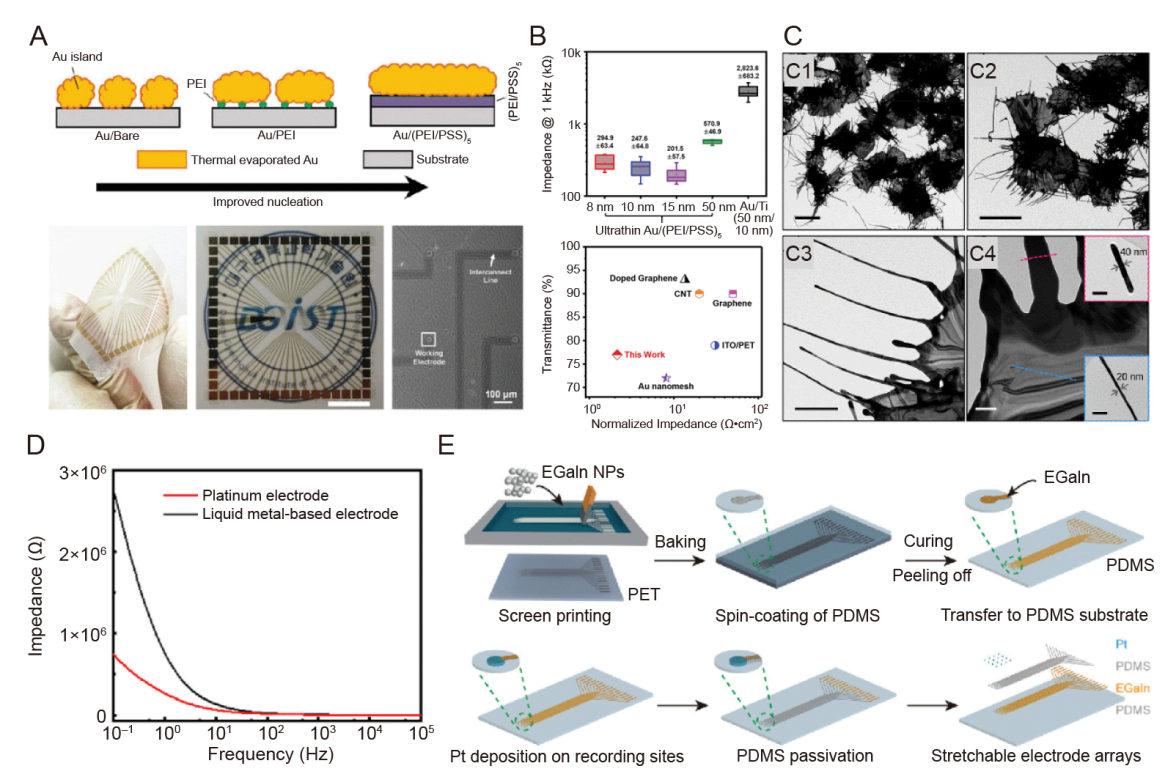
Figure 1. New metal material electrode. (A) Schematic of the formation process of ultrathin Au electrodes on polyelectrolyte coatings and photographic and microscopic images of Au/(PEI/PSS)5 MEA on plastic. (B) The magnitude of the impedance at 1 kHz (top) and a comparison of the area-normalised electrochemical impedance and light transmittance in a recently developed neuro-microelectrode (bottom). A and B were reprinted from Hong et al.67 Copyright 2022 Wiley‐VCH GmbH. Reproduced with permission. (C) TEM images of whiskered Au nanosheets showing the overall morphology and magnified views of the edge portion of the whiskers. Scale bars: 5 μm (C1, C2), 1 μm (C3), 200 nm (C4). Reprinted with permission from Lim et al.68 Copyright 2022 American Chemical Society. (D) The EIS curves of the LM-based and Pt electrodes under 1 × 10-1 up to 1 × 105 Hz at a scan rate of 0.1 V/s (in normal saline, 10 mV sine wave).71 (E) Fabrication of stretchable metal electrodes based on liquid metal-polymer conductors using screen printing, which reprinted from Dong et al.69 Copyright 2021 Wiley‐VCH GmbH. Reproduced with permission. Au: aurum; EGaIn: eutectic gallium-indium alloy; EIS: electrochemical impedance spectroscopy; LM: liquid metal; MEA: microelectrode array; NP: nanoparticle; PDMS: poly(dimethylsiloxane); PEI: polyethylenimine; PET: polyethylene terephthalate; PSS: poly(styrene sulfonate); Pt: platinum; TEM: transmission electron microscopy.
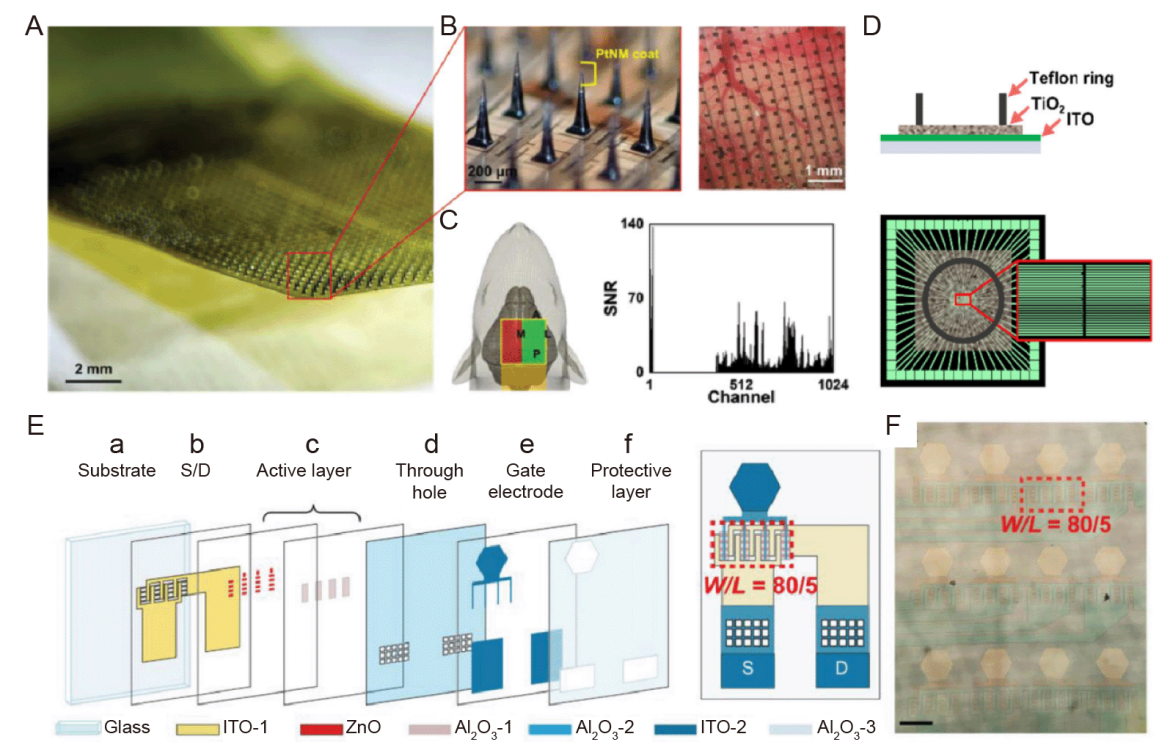
Figure 2. Semiconductor materials neuroelectrodes. (A) Photograph of a 1024-channel SiMNA and a magnified view of an array with a tapered SiMN with a height of approximately 300 μm and a tip coated with PtNM. Scale bar: 2 mm. (B) Magnified view of a 1024-channel SiMNA implanted in the right hemisphere of the rat brain. Scale bar: 200 μm (left), 1 mm (right). (C) Schematic diagram of SiMNA implantation in the right hemisphere with electrical connections pointing toward the back of the rat (green highlighted area indicates successful implantation of cortical SiMNs, while the SiMN in the red highlighted area is located at the top of the rat skull) and a signal-to-noise histogram of whisker blowing stimulation evoked LFP response. A-C were reprinted from Lee et al.75 Copyright 2022 Wiley‐VCH GmbH. Reproduced with permission. (D) Side and top views of patterned TiO2 electrodes. The green parallel lines in the top view represent ITO patterns attached to 60 external contact pads.78 (E) Left: (a, b) The first ITO layer of 100-nm thickness for source and drain, (c) 20-nm thick ZnO active layer and the first Al2O3 layer of 10-nm thickness for the ZnO layer protection, (d) the second Al2O3 layer of 20-nm thickness as the gate dielectric layer and through-holes punched in the layer, (e) the second ITO layer of 100-nm thickness for the gate electrodes, and (f) the third Al2O3 layer of 20-nm thickness to protect the whole device. Right: Structure of a ZnO-TFT electrode. The red dashed square labels a transistor with W/L = 80/5, which consist of 16 transistors with W/L = 5/5 in parallel. (F) Microscopic image of a 3 × 4 ZnO-TFT array. The red dashed frame labels an active region consisting of 16 paralleled ZnO-TFT, corresponding to that in the red dashed frame in E (right). Scale bar: 200 μm. E and F were reprinted from Zhang et al.81 Al2O3: aluminum oxide; D: drain; ITO: indium-tin-oxide; LFP: local field potential; PtNM: platinum nanomesh; S: source; SiMNA: silicon microneedle array; SNR: signal-to-noise ratio; TFT: thin-film transistor; TiO2: titanium dioxide; W/L: width-to-length ratio; ZnO: zinc oxide.
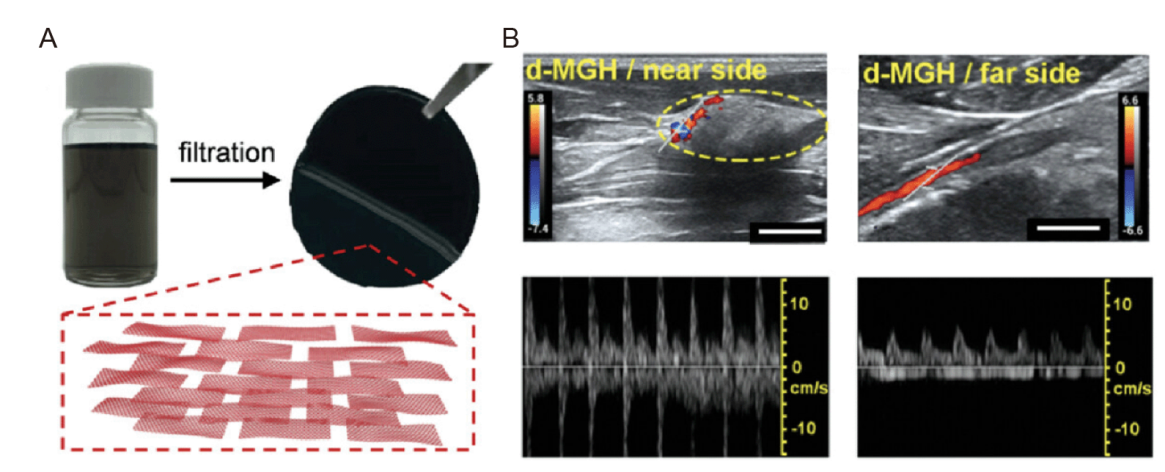
Figure 3. Neuroelectrodes are made of carbon nanomaterials. (A) Photograph of graphene dispersion and MGH membrane (top). The inset (bottom) outlines the multilayer structure of the MGH membrane made of CCG nanosheets (red).87 (B) Proximal and distal d-MGH at 8 weeks after implantation. The color bars represent the blood flow velocity. Scale bars: 5.0 mm (left), 2.0 mm (right).87 CCG: chemically converted graphene; d-MGH: CCG nanosheets densely packed MGH; MGH: multilayer graphene hydrogel.
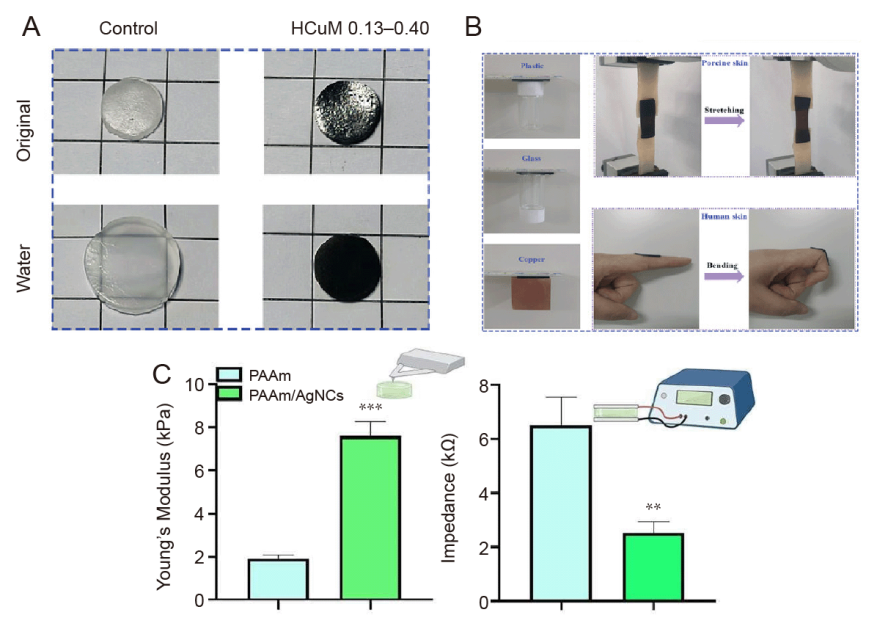
Figure 4. Neuroelectrodes are made of conductive polymer materials. (A) Swelling study of pNIPAm hydrogel and HCuM 0.13–0.40 in water for 5 days. (B) Demonstration of adhesion of HCuM 0.13–0.40. A and B were reprinted from Hong et al.94 Copyright 2022 Wiley‐VCH GmbH. Reproduced with permission. (C) Histogram of Young’s modulus, showing the mechanical properties of PAAm/AgNCs compared to PAAm hydrogels, measured by nanoindentation method (left) and histogram of the electrical impedance values, measured at a physiologically significant frequency (10 Hz), showing the superior electrical properties of the PAAm/AgNCs hydrogel (right). Data are expressed as mean ± SD. **P ≤ 0.01, **P ≤ 0.001. Reprinted with permission from Rinoldi et al.95 Copyright 2023 American Chemical Society. AgNC: silver nanocube; HCuM: a set of polymer network hydrogels interpenetrated with poly(N-isopropylacrylamide) and poly(Cu-arylacetyl); PAAm: polyacrylamide.
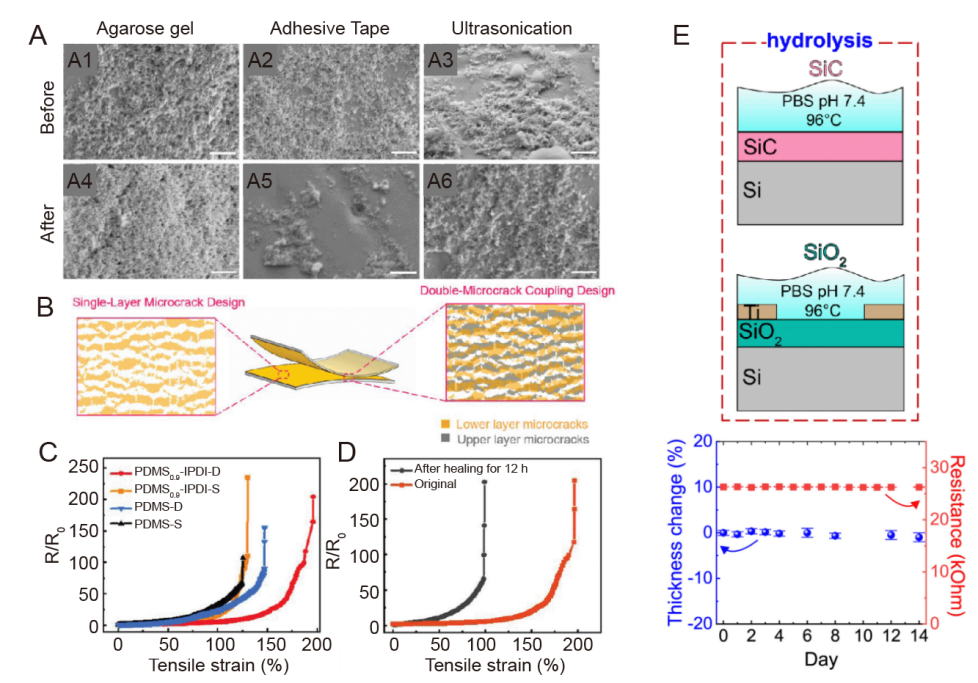
Figure 5. Coating of metallic materials for neural electrodes. (A) SEM images of the side of the PDC-coated neural electrode before (A1-3) and after (A4-6) mechanical stability tests. Scale bars: 500 nm.123 (B) Schematic diagrams of stretchable electrodes based on a single-layer microcrack design and a dual microcrack-coupled design. (C) R/R0 versus tensile strain for Au electrodes based on PDMS 0.9-IPDI and PDMS substrates, respectively (-D for a double-layer microcrack, -S for a single-layer microcrack). (D) Dual microcrack-coupled PDMS 0.9-IPDI electrodes Change in tensile resistance after 12 hours of healing at 25°C. B-D were reprinted from Yang et al.126 Copyright 2023 Wiley‐VCH GmbH. Reproduced with permission. (E) Soaking test of hydrolysis in SiC and as-grown SiO2 in 1× PBS at different temperatures up to 96°C (top) and SiC thickness and electrical resistance variations after the accelerated hydrolysis test in PBS at 96°C after up to 14 days (bottom).127 Au: aurum; IPDI: isophorone diisocyanate; PBS: phosphate buffer solution; PDC: pulsed direct current; PDMS: poly(dimethylsiloxane); R/R0: resistance change; SEM: scanning electron microscope; SiC: silicon carbide; SiO2: silicon dioxide; Ti: titanium.
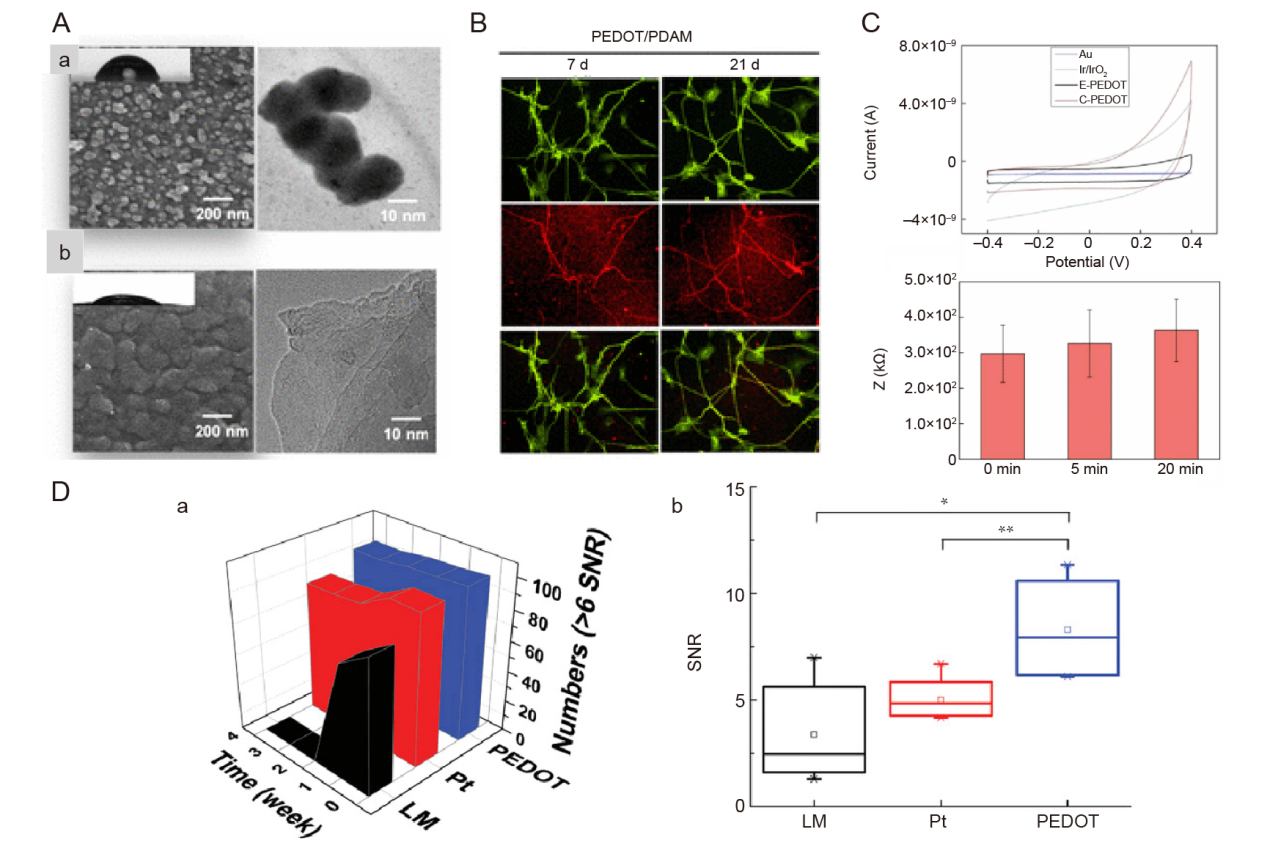
Figure 6. Conductive polymer material coatings for nerve electrodes. (A) SEM and TEM images of (a) PEDOT and (b) PEDOT/PDAM films. Scale bars: 200 nm (left), 10 nm (right). (B) Fluorescence images showing immunofluorescence staining of β-tubulin III (green), MAP2 (red), and nuclei (blue) after culturing neuronal progenitor cells in PEDOT/PDAM films for 21 days. (C) Cyclic voltammetry curves of chemically polymerised PEDOT and other interfaces (n = 3), impedance of electrodes at 20 kHz frequency after sonication. A-C were reprinted with permission from Huang et al.130 Copyright 2022 American Chemical Society. (D) (a) The numbers of high-quality recording spikes (SNR > 6) of LM wire, Pt wire, and PEDOT LMEs for 4 weeks, and (b) analysis of variance test revealed the significant difference among the electrodes. *P < 0.05, **P < 0.01. D was reprinted from Lim et al.133 Copyright 2022 Wiley‐VCH GmbH. Reproduced with permission. Au: aurum; C-PEDOT: chemical polymerization PEDOT; E-PEDOT: electropolymerization PEDOT; Ir: iridium; IrO3: iridium trioxide; LM: liquid metal; LME: liquid metal based electrode; MAP2: microtubule-associated protein 2; PDAM: 1-pyrenyldiazomethane; PEDOT: poly(3,4-ethylenedioxythiophene); Pt: platinum; SEM: scanning electron microscope; SNR: signal-to-noise ratio; TEM: transmission electron microscope.
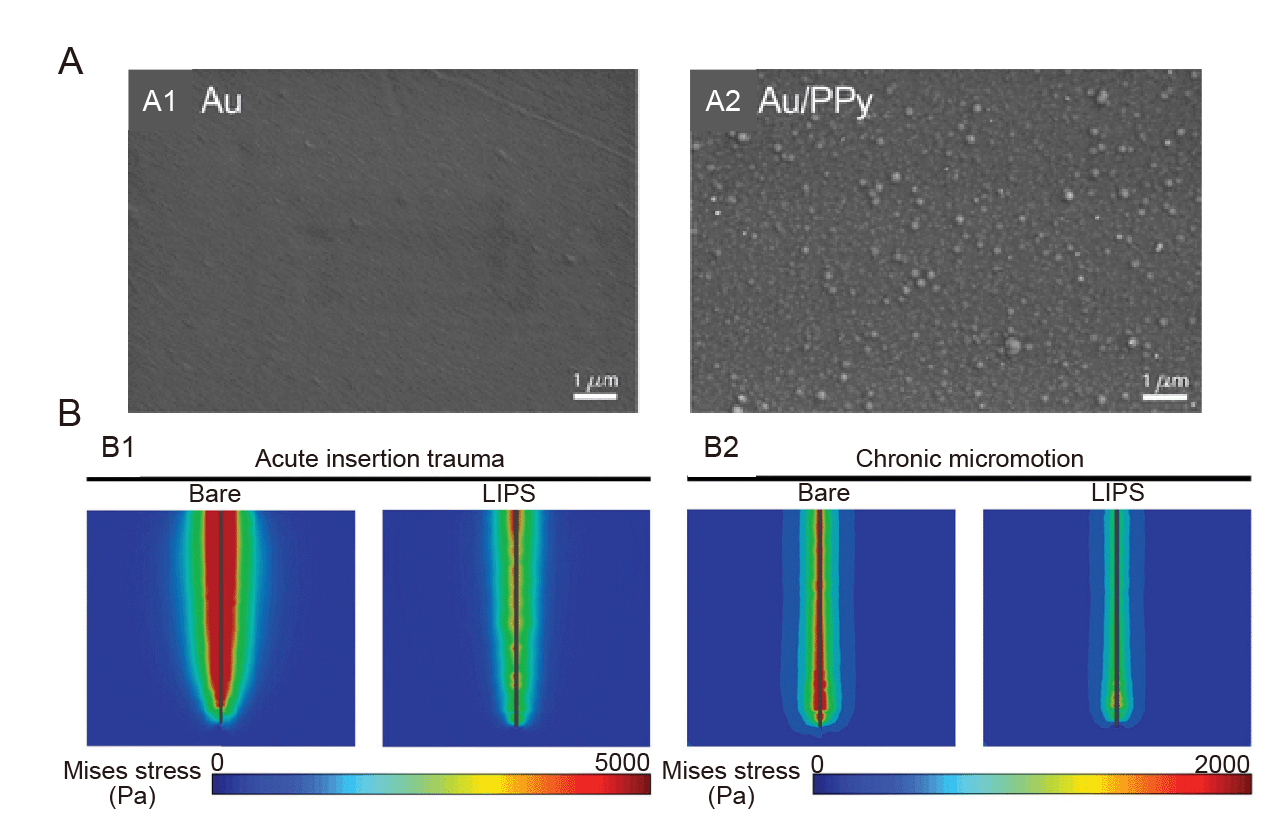
Figure 7. Coating with active substance grafts. (A) SEM images of (Aa) Au polyester film electrode and (A2) Au/PPy electrode surface. Reprinted with permission from Desroches et al.138 Copyright 2020 American Chemical Society. (B) von Mises stress profiles of bare and LIPS-coated probes within brain tissue during insertion (B1) and under lateral micromotion of 100 μm (B2).141 Au: aurum; LIPS: lubricated immune-stealthy probe surface; PPy: polypyrrole.
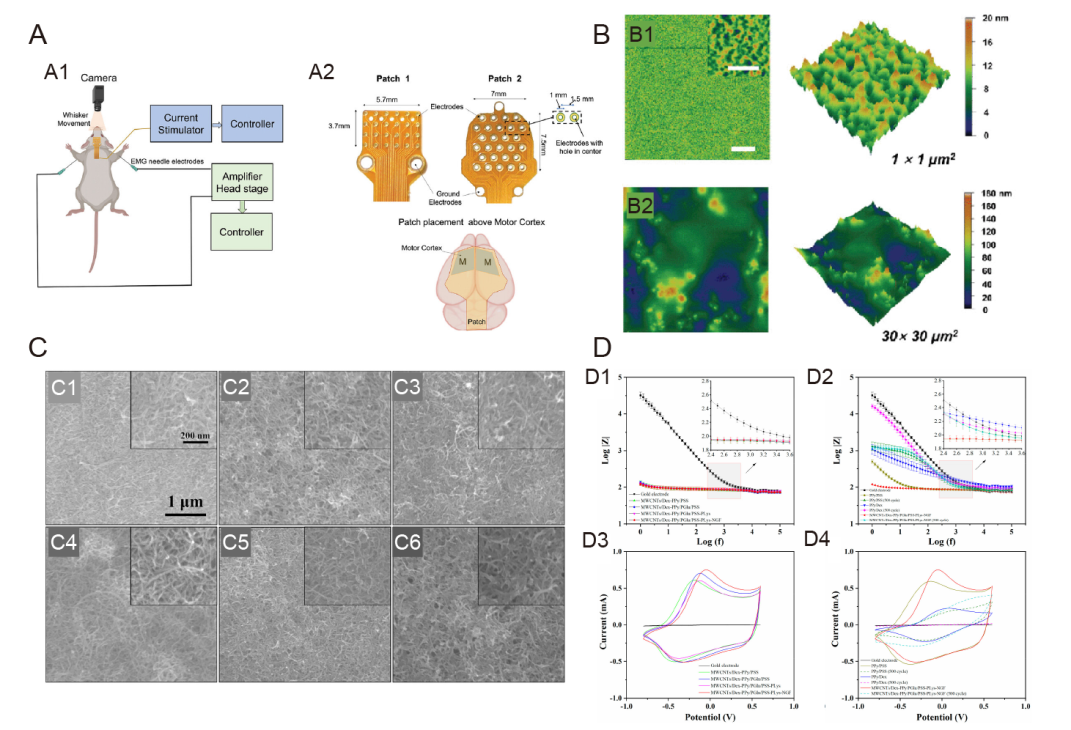
Figure 8. Neuroelectrodes with composite coatings. (A) (A1) Schematic representation of stimulation paradigm showing connection of the stimulation patch and EMG electrodes placement along with camera for whisker movement recording. (A2) Two different flexible patches were used for HD-ECS. Patch 1 contained 24 disk electrodes arranged in a 6 × 4 array in a cartesian grid, and Patch 2 had 27 ring-shaped electrodes arranged in a hexagonal grid. The patch was placed above the skull with its centre aligned to bregma, covering the motor cortex of both hemispheres.142 (B) Surface morphology (2D and 3D height images) of c-PEG-0 (B1) and c-PEG-20 (B2) samples in water taken by AFM. Scale bars: 5 μm (inset: 500 nm).146 (C) SEM images of different functional coatings. (C1) MWCNTs, (C2) MWCNTs/Dex-PPy/PSS, (C3) MWCNTs/Dex-PPy/PGlu/PSS, (C4) MWCNTs/Dex-PPy/PGlu/PSS-PLys, (C5) MWCNTs/Dex-PPy/PGlu/PSS-PLys-NGF (before CV stimulation), and (C6) MWCNTs/Dex-PPy/PGlu/PSS-PLys-NGF coatings (after 500 cycles of CV stimulation). Insets are corresponding images with higher magnification. (D) (D1, D3) EIS (D1) and CV (D3) of bare Au electrodes, MWCNTs/Dex-PPy/PSS, MWCNTs/Dex-PPy/PGlu/PSS, MWCNTs/Dex-PPy/PGlu/PSS-PLys, and MWCNTs/Dex-PPy/PGlu/PSS-PLys-NGF functional coatings. Comparison of EIS (D2) and CV (D4) of bare Au electrodes, PPy/PSS films, PPy/Dex films, and MWCNTs/Dex-PPy/PGlu/PSS-PLys-NGF functional coatings. Data are represented by mean ± standard deviation (n ≥ 3). Reprinted with permission from Tian et al.147 Copyright 2022 American Chemical Society. 2D: two dimensional; 3D: three dimensional; AFM: atomic force microscope; Au: aurum; c-PEG: carbon nanotube-poly(ethylene glycol); CV: cyclic voltammetry; Dex: dexamethasone; MWCNT: multiwalled carbon nanotube; NGF: nerve growth factor; PGlu: poly(glutamic acid); PLys: poly(lysine); PPy: polypyrrole; PSS: poly(styrene sulfonate); SEM: scanning electron microscope.
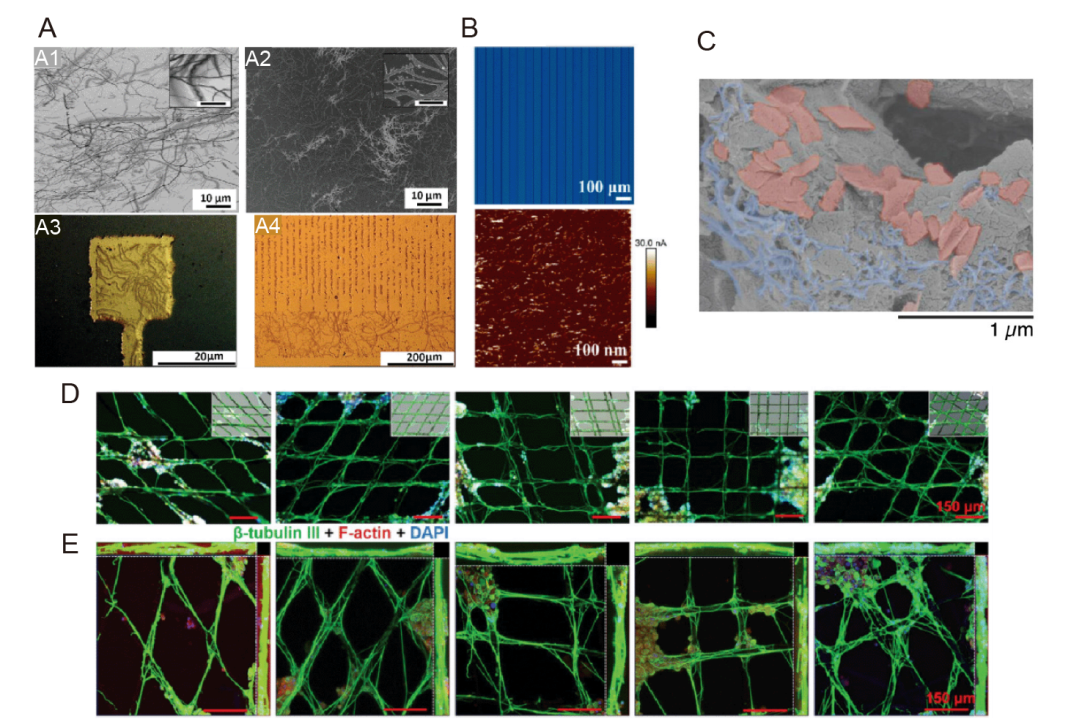
Figure 9. Neuroelectrode coatings for regulating cellular activity through microforms. (A) SEM images of (A1) collagen fibres coated on silicon wafer and (A2) collagen-like Au nanostructures developed from the nanoimprint process; Application of CLGNS nanostructuring process on (A3) microelectrode array surface and (A4) meander pattern, as proof of concept.192 Scale bars: 10 μm (A1, A2), 20 μm (A3, A4) (inset scale bar 1 μm). (B) Photograph of photopatterned MH (15 wt% SBMA) and AFM current image of PEDOT:PSS treated with SBMA (15 wt%). Reprinted with permission from Yang et al.58 Copyright 2023 American Chemical Society. (C) SEM micrographs of the scaffolds with alginate left gray, GF pseudo-colored red, and CNTs pseudo-colored blue. Scale bar: 1 μm. C was reprinted from Tringides et al.193 Copyright 2023 Wiley‐VCH GmbH. (D) Representative immunofluorescence images and (E) confocal microscopy images of PC-12 cells cultured on microfibres with different cladding angles for 14 days under ES conditions. Cultures were immunofluorescently stained with β-tubulin III (green), F-actin (red) and DAPI (blue). Inset: merged bright field images. D was reprinted from Wang et al.194 Copyright 2020 Wiley‐VCH GmbH. Scale bars: 150 μm. AFM: atomic force microscope; CLGNS: collagen-like gold nanostructure; DAPI: 4,6-diamidino-2-phenylindole; ES: electrical stimulation; MH: multifunctional hydrogel; PEDOT:PSS: poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate); SBMA: 3-[dimethyl-[2-(2-methylprop-2-enoyloxy)ethyl] azaniumyl] propane-1-sulfonate; SEM: scanning electron microscope.
| 1. |
Serino, A.; Bockbrader, M.; Bertoni, T.; Colachis Iv, S.; Solcà, M.; Dunlap, C.; Eipel, K.; Ganzer, P.; Annetta, N.; Sharma, G.; Orepic, P.; Friedenberg, D.; Sederberg, P.; Faivre, N.; Rezai, A.; Blanke, O. Sense of agency for intracortical brain-machine interfaces. Nat Hum Behav. 2022, 6, 565-578.
doi: 10.1038/s41562-021-01233-2 |
| 2. |
Shanechi, M. M. Brain-machine interfaces from motor to mood. Nat Neurosci. 2019, 22, 1554-1564.
doi: 10.1038/s41593-019-0488-y |
| 3. |
Young, M. J.; Lin, D. J.; Hochberg, L. R. Brain-Computer Interfaces in Neurorecovery and Neurorehabilitation. Semin Neurol. 2021, 41, 206-216.
doi: 10.1055/s-0041-1725137 URL |
| 4. |
Steins, H.; Mierzejewski, M.; Brauns, L.; Stumpf, A.; Kohler, A.; Heusel, G.; Corna, A.; Herrmann, T.; Jones, P. D.; Zeck, G.; von Metzen, R.; Stieglitz, T. A flexible protruding microelectrode array for neural interfacing in bioelectronic medicine. Microsyst Nanoeng. 2022, 8, 131.
doi: 10.1038/s41378-022-00466-z |
| 5. |
Choi, J. S.; Lee, H. J.; Rajaraman, S.; Kim, D. H. Recent advances in three-dimensional microelectrode array technologies for in vitro and in vivo cardiac and neuronal interfaces. Biosens Bioelectron. 2021, 171, 112687.
doi: 10.1016/j.bios.2020.112687 URL |
| 6. |
Fattahi, P.; Yang, G.; Kim, G.; Abidian, M. R. A review of organic and inorganic biomaterials for neural interfaces. Adv Mater. 2014, 26, 1846-1885.
doi: 10.1002/adma.v26.12 URL |
| 7. |
Cruz, A. M.; Casañ-Pastor, N. Graded conducting titanium-iridium oxide coatings for bioelectrodes in neural systems. Thin Solid Films. 2013, 534, 316-324.
doi: 10.1016/j.tsf.2013.02.031 URL |
| 8. |
Chapman, C. A.; Chen, H.; Stamou, M.; Biener, J.; Biener, M. M.; Lein, P. J.; Seker, E. Nanoporous gold as a neural interface coating: effects of topography, surface chemistry, and feature size. ACS Appl Mater Interfaces. 2015, 7, 7093-7100.
doi: 10.1021/acsami.5b00410 URL |
| 9. |
Boehler, C.; Stieglitz, T.; Asplund, M. Nanostructured platinum grass enables superior impedance reduction for neural microelectrodes. Biomaterials. 2015, 67, 346-353.
doi: 10.1016/j.biomaterials.2015.07.036 URL |
| 10. |
Li, J.; Cheng, Y.; Gu, M.; Yang, Z.; Zhan, L.; Du, Z. Sensing and stimulation applications of carbon nanomaterials in implantable brain-computer interface. Int J Mol Sci. 2023, 24, 5182.
doi: 10.3390/ijms24065182 URL |
| 11. |
Kim, T.; Park, J.; Sohn, J.; Cho, D.; Jeon, S. Bioinspired, highly stretchable, and conductive dry adhesives based on 1D-2D hybrid carbon nanocomposites for all-in-one ECG electrodes. ACS Nano. 2016, 10, 4770-4778.
doi: 10.1021/acsnano.6b01355 URL |
| 12. |
Kuzum, D.; Takano, H.; Shim, E.; Reed, J. C.; Juul, H.; Richardson, A. G.; de Vries, J.; Bink, H.; Dichter, M. A.; Lucas, T. H.; Coulter, D. A.; Cubukcu, E.; Litt, B. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat Commun. 2014, 5, 5259.
doi: 10.1038/ncomms6259 |
| 13. |
Golparvar, A. J.; Yapici, M. K. Electrooculography by wearable graphene textiles. IEEE Sens J. 2018, 18, 8971-8978.
doi: 10.1109/JSEN.2018.2868879 URL |
| 14. |
Apollo, N. V.; Maturana, M. I.; Tong, W.; Nayagam, D. A. X.; Shivdasani, M. N.; Foroughi, J.; Wallace, G. G.; Prawer, S.; Ibbotson, M. R.; Garrett, D. J. Soft, Flexible freestanding neural stimulation and recording electrodes fabricated from reduced graphene oxide. Adv Funct Mater. 2015, 25, 3551-3559.
doi: 10.1002/adfm.v25.23 URL |
| 15. |
Du, X.; Jiang, W.; Zhang, Y.; Qiu, J.; Zhao, Y.; Tan, Q.; Qi, S.; Ye, G.; Zhang, W.; Liu, N. Transparent and stretchable graphene electrode by intercalation doping for epidermal electrophysiology. ACS Appl Mater Interfaces. 2020, 12, 56361-56371.
doi: 10.1021/acsami.0c17658 URL |
| 16. |
Green, R.; Abidian, M. R. Conducting polymers for neural prosthetic and neural interface applications. Adv Mater. 2015, 27, 7620-7637.
doi: 10.1002/adma.v27.46 URL |
| 17. | Green, R. A.; Lovell, N. H.; Wallace, G. G.; Poole-Warren, L. A. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials. 2008, 29, 3393-3399. |
| 18. |
Guo, L.; Ma, M.; Zhang, N.; Langer, R.; Anderson, D. G. Stretchable polymeric multielectrode array for conformal neural interfacing. Adv Mater. 2014, 26, 1427-1433.
doi: 10.1002/adma.v26.9 URL |
| 19. |
Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C. W.; Tang, Y.; Weng, L. T.; Yuan, H. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small. 2017, 13, 1601916.
doi: 10.1002/smll.v13.2 URL |
| 20. |
Kim, D. H.; Viventi, J.; Amsden, J. J.; Xiao, J.; Vigeland, L.; Kim, Y. S.; Blanco, J. A.; Panilaitis, B.; Frechette, E. S.; Contreras, D.; Kaplan, D. L.; Omenetto, F. G.; Huang, Y.; Hwang, K. C.; Zakin, M. R.; Litt, B.; Rogers, J. A. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat Mater. 2010, 9, 511-517.
doi: 10.1038/nmat2745 |
| 21. |
Adewole, D. O.; Serruya, M. D.; Wolf, J. A.; Cullen, D. K. Bioactive neuroelectronic interfaces. Front Neurosci. 2019, 13, 269.
doi: 10.3389/fnins.2019.00269 URL |
| 22. |
Chalmers, E.; Lee, H.; Zhu, C.; Liu, X. Increasing the conductivity and adhesion of polypyrrole hydrogels with electropolymerized polydopamine. Chem Mater. 2020, 32, 234-244.
doi: 10.1021/acs.chemmater.9b03655 URL |
| 23. |
Gajendiran, M.; Choi, J.; Kim, S. J.; Kim, K.; Shin, H.; Koo, H. J.; Kim, K. Conductive biomaterials for tissue engineering applications. J Ind Eng Chem. 2017, 51, 12-26.
doi: 10.1016/j.jiec.2017.02.031 URL |
| 24. |
Franze, K.; Janmey, P. A.; Guck, J. Mechanics in neuronal development and repair. Annu Rev Biomed Eng. 2013, 15, 227-251.
doi: 10.1146/bioeng.2013.15.issue-1 URL |
| 25. |
Budday, S.; Ovaert, T. C.; Holzapfel, G. A.; Steinmann, P.; Kuhl, E. Fifty shades of brain: a review on the mechanical testing and modeling of brain tissue. Arch Comput Methods Eng. 2020, 27, 1187-1230.
doi: 10.1007/s11831-019-09352-w |
| 26. |
Betz, T.; Koch, D.; Lu, Y. B.; Franze, K.; Käs, J. A. Growth cones as soft and weak force generators. Proc Natl Acad Sci U S A. 2011, 108, 13420-13425.
doi: 10.1073/pnas.1106145108 URL |
| 27. |
Zamproni, L. N.; Mundim, M.; Porcionatto, M. A. Neurorepair and regeneration of the brain: a decade of bioscaffolds and engineered microtissue. Front Cell Dev Biol. 2021, 9, 649891.
doi: 10.3389/fcell.2021.649891 URL |
| 28. |
Carnicer-Lombarte, A.; Chen, S. T.; Malliaras, G. G.; Barone, D. G. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. Front Bioeng Biotechnol. 2021, 9, 622524.
doi: 10.3389/fbioe.2021.622524 URL |
| 29. |
Michelson, N. J.; Vazquez, A. L.; Eles, J. R.; Salatino, J. W.; Purcell, E. K.; Williams, J. J.; Cui, X. T.; Kozai, T. D. Y. Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: new emphasis on the biological interface. J Neural Eng. 2018, 15, 033001.
doi: 10.1088/1741-2552/aa9dae URL |
| 30. |
Bennett, C.; Mohammed, F.; Álvarez-Ciara, A.; Nguyen, M. A.; Dietrich, W. D.; Rajguru, S. M.; Streit, W. J.; Prasad, A. Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamine as an iron chelator on acute foreign body response. Biomaterials. 2019, 188, 144-159.
doi: 10.1016/j.biomaterials.2018.09.040 URL |
| 31. |
Kozai, T. D.; Vazquez, A. L.; Weaver, C. L.; Kim, S. G.; Cui, X. T. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J Neural Eng. 2012, 9, 066001.
doi: 10.1088/1741-2560/9/6/066001 URL |
| 32. |
Wellman, S. M.; Cambi, F.; Kozai, T. D. The role of oligodendrocytes and their progenitors on neural interface technology: A novel perspective on tissue regeneration and repair. Biomaterials. 2018, 183, 200-217.
doi: 10.1016/j.biomaterials.2018.08.046 URL |
| 33. |
Biran, R.; Martin, D. C.; Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005, 195, 115-126.
doi: 10.1016/j.expneurol.2005.04.020 URL |
| 34. |
Chen, K.; Wellman, S. M.; Yaxiaer, Y.; Eles, J. R.; Kozai, T. D. In vivo spatiotemporal patterns of oligodendrocyte and myelin damage at the neural electrode interface. Biomaterials. 2021, 268, 120526.
doi: 10.1016/j.biomaterials.2020.120526 URL |
| 35. |
Savya, S. P.; Li, F.; Lam, S.; Wellman, S. M.; Stieger, K. C.; Chen, K.; Eles, J. R.; Kozai, T. D. Y. In vivo spatiotemporal dynamics of astrocyte reactivity following neural electrode implantation. Biomaterials. 2022, 289, 121784.
doi: 10.1016/j.biomaterials.2022.121784 URL |
| 36. |
Kim, G. H.; Kim, K.; Nam, H.; Shin, K.; Choi, W.; Shin, J. H.; Lim, G. CNT-Au nanocomposite deposition on gold microelectrodes for improved neural recordings. Sens Actuators B Chem. 2017, 252, 152-158.
doi: 10.1016/j.snb.2017.04.142 URL |
| 37. |
Yuan, X.; Hierlemann, A.; Frey, U. Extracellular recording of entire neural networks using a dual-mode microelectrode array with 19584 electrodes and high SNR. IEEE J Solid-State Circuits. 2021, 56, 2466-2475.
doi: 10.1109/JSSC.2021.3066043 URL |
| 38. |
Lin, C. M.; Lee, Y. T.; Yeh, S. R.; Fang, W. Flexible carbon nanotubes electrode for neural recording. Biosens Bioelectron. 2009, 24, 2791-2797.
doi: 10.1016/j.bios.2009.02.005 URL |
| 39. |
Sabetian, P.; Popovic, M. R.; Yoo, P. B. Optimizing the design of bipolar nerve cuff electrodes for improved recording of peripheral nerve activity. J Neural Eng. 2017, 14, 036015.
doi: 10.1088/1741-2552/aa6407 URL |
| 40. |
Díaz, D. R.; Carmona, F. J.; Palacio, L.; Ochoa, N. A.; Hernández, A.; Prádanos, P. Impedance spectroscopy and membrane potential analysis of microfiltration membranes. The influence of surface fractality. Chem Eng Sci. 2018, 178, 27-38.
doi: 10.1016/j.ces.2017.12.027 URL |
| 41. |
Wellman, S. M.; Li, L.; Yaxiaer, Y.; McNamara, I.; Kozai, T. D. Y. Revealing spatial and temporal patterns of cell death, glial proliferation, and blood-brain barrier dysfunction around implanted intracortical neural interfaces. Front Neurosci. 2019, 13, 493.
doi: 10.3389/fnins.2019.00493 URL |
| 42. |
Wellman, S. M.; Kozai, T. D. Y. In vivo spatiotemporal dynamics of NG2 glia activity caused by neural electrode implantation. Biomaterials. 2018, 164, 121-133.
doi: 10.1016/j.biomaterials.2018.02.037 URL |
| 43. |
Camuñas-Mesa, L. A.; Quiroga, R. Q. A detailed and fast model of extracellular recordings. Neural Comput. 2013, 25, 1191-1212.
doi: 10.1162/NECO_a_00433 URL |
| 44. |
Kozai, T. D.; Langhals, N. B.; Patel, P. R.; Deng, X.; Zhang, H.; Smith, K. L.; Lahann, J.; Kotov, N. A.; Kipke, D. R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater. 2012, 11, 1065-1073.
doi: 10.1038/nmat3468 |
| 45. |
Lee, H. C.; Ejserholm, F.; Gaire, J.; Currlin, S.; Schouenborg, J.; Wallman, L.; Bengtsson, M.; Park, K.; Otto, K. J. Histological evaluation of flexible neural implants; flexibility limit for reducing the tissue response? J Neural Eng. 2017, 14, 036026.
doi: 10.1088/1741-2552/aa68f0 URL |
| 46. |
Seymour, J. P.; Kipke, D. R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007, 28, 3594-3607.
doi: 10.1016/j.biomaterials.2007.03.024 URL |
| 47. |
Kuo, J. T.; Kim, B. J.; Hara, S. A.; Lee, C. D.; Gutierrez, C. A.; Hoang, T. Q.; Meng, E. Novel flexible parylene neural probe with 3D sheath structure for enhancing tissue integration. Lab Chip. 2013, 13, 554-561.
doi: 10.1039/C2LC40935F URL |
| 48. |
Gao, K.; Li, G.; Liao, L.; Cheng, J.; Zhao, J.; Xu, Y. Fabrication of flexible microelectrode arrays integrated with microfluidic channels for stable neural interfaces. Sens Actuators A Phys. 2013, 197, 9-14.
doi: 10.1016/j.sna.2013.04.005 URL |
| 49. |
Du, Z. J.; Kolarcik, C. L.; Kozai, T. D. Y.; Luebben, S. D.; Sapp, S. A.; Zheng, X. S.; Nabity, J. A.; Cui, X. T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46-58.
doi: 10.1016/j.actbio.2017.02.010 URL |
| 50. |
Hong, G.; Lieber, C. M. Novel electrode technologies for neural recordings. Nat Rev Neurosci. 2019, 20, 330-345.
doi: 10.1038/s41583-019-0140-6 |
| 51. | Zhu, M.; Wang, H.; Li, S.; Liang, X.; Zhang, M.; Dai, X.; Zhang, Y. Flexible electrodes for in vivo and in vitro electrophysiological signal recording. Adv Healthc Mater. 2021, 10, e2100646. |
| 52. |
Hu, Z.; Niu, Q.; Hsiao, B. S.; Yao, X.; Zhang, Y. Bioactive polymer-enabled conformal neural interface and its application strategies. Mater Horiz. 2023, 10, 808-828.
doi: 10.1039/D2MH01125E URL |
| 53. |
Potter-Baker, K. A.; Nguyen, J. K.; Kovach, K. M.; Gitomer, M. M.; Srail, T. W.; Stewart, W. G.; Skousen, J. L.; Capadona, J. R. Development of superoxide dismutase mimetic surfaces to reduce accumulation of reactive oxygen species for neural interfacing applications. J Mater Chem B. 2014, 2, 2248-2258.
doi: 10.1039/C4TB00125G URL |
| 54. |
Zheng, X. S.; Snyder, N. R.; Woeppel, K.; Barengo, J. H.; Li, X.; Eles, J.; Kolarcik, C. L.; Cui, X. T. A superoxide scavenging coating for improving tissue response to neural implants. Acta Biomater. 2019, 99, 72-83.
doi: 10.1016/j.actbio.2019.08.032 URL |
| 55. |
Golabchi, A.; Wu, B.; Li, X.; Carlisle, D. L.; Kozai, T. D. Y.; Friedlander, R. M.; Cui, X. T. Melatonin improves quality and longevity of chronic neural recording. Biomaterials. 2018, 180, 225-239.
doi: 10.1016/j.biomaterials.2018.07.026 URL |
| 56. | Zhang, J.; Wang, L.; Xue, Y.; Lei, I. M.; Chen, X.; Zhang, P.; Cai, C.; Liang, X.; Lu, Y.; Liu, J. Engineering electrodes with robust conducting hydrogel coating for neural recording and modulation. Adv Mater. 2023, 35, e2209324. |
| 57. |
Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem Soc Rev. 2019, 48, 1642-1667.
doi: 10.1039/C8CS00595H URL |
| 58. | Yang, M.; Chen, P.; Qu, X.; Zhang, F.; Ning, S.; Ma, L.; Yang, K.; Su, Y.; Zang, J.; Jiang, W.; Yu, T.; Dong, X.; Luo, Z. Robust neural interfaces with photopatternable, bioadhesive, and highly conductive hydrogels for stable chronic neuromodulation. ACS Nano. 2023. doi: 10.1021/acsnano.2c04606. |
| 59. |
Wu, Z. Z.; Zhao, Y.; Kisaalita, W. S. Interfacing SH-SY5Y human neuroblastoma cells with SU-8 microstructures. Colloids Surf B Biointerfaces. 2006, 52, 14-21.
doi: 10.1016/j.colsurfb.2006.06.001 URL |
| 60. |
Fan, Y. W.; Cui, F. Z.; Hou, S. P.; Xu, Q. Y.; Chen, L. N.; Lee, I. S. Culture of neural cells on silicon wafers with nano-scale surface topograph. J Neurosci Methods. 2002, 120, 17-23.
doi: 10.1016/S0165-0270(02)00181-4 URL |
| 61. |
Kushwah, N.; Woeppel, K.; Dhawan, V.; Shi, D.; Cui, X. T. Effects of neuronal cell adhesion molecule L1 and nanoparticle surface modification on microglia. Acta Biomater. 2022, 149, 273-286.
doi: 10.1016/j.actbio.2022.06.038 URL |
| 62. | Woeppel, K. M.; Cui, X. T. Nanoparticle and biomolecule surface modification synergistically increases neural electrode recording yield and minimizes inflammatory host response. Adv Healthc Mater. 2021, 10, e2002150. |
| 63. |
Sikder, M. K. U.; Tong, W.; Pingle, H.; Kingshott, P.; Needham, K.; Shivdasani, M. N.; Fallon, J. B.; Seligman, P.; Ibbotson, M. R.; Prawer, S.; Garrett, D. J. Laminin coated diamond electrodes for neural stimulation. Mater Sci Eng C Mater Biol Appl. 2021, 118, 111454.
doi: 10.1016/j.msec.2020.111454 URL |
| 64. |
Chou, N.; Byun, D.; Kim, S. MEMS-based microelectrode technologies capable of penetrating neural tissues. Biomed Eng Lett. 2014, 4, 109-119.
doi: 10.1007/s13534-014-0133-3 URL |
| 65. | Trevathan, J. K.; Baumgart, I. W.; Nicolai, E. N.; Gosink, B. A.; Asp, A. J.; Settell, M. L.; Polaconda, S. R.; Malerick, K. D.; Brodnick, S. K.; Zeng, W.; Knudsen, B. E.; McConico, A. L.; Sanger, Z.; Lee, J. H.; Aho, J. M.; Suminski, A. J.; Ross, E. K.; Lujan, J. L.; Weber, D. J.; Williams, J. C.; Franke, M.; Ludwig, K. A.; Shoffstall, A. J. An injectable neural stimulation electrode made from an in-body curing polymer/metal composite. Adv Healthc Mater. 2019, 8, e1900892. |
| 66. |
Patel, P. R.; Zhang, H.; Robbins, M. T.; Nofar, J. B.; Marshall, S. P.; Kobylarek, M. J.; Kozai, T. D.; Kotov, N. A.; Chestek, C. A. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. J Neural Eng. 2016, 13, 066002.
doi: 10.1088/1741-2560/13/6/066002 URL |
| 67. |
Hong, W.; Lee, J. W.; Kim, D.; Hwang, Y.; Lee, J.; Kim, J.; Hong, N.; Kwon, H. J.; Jang, J. E.; Punga, A. R.; Kang, H. Ultrathin gold microelectrode array using polyelectrolyte multilayers for flexible and transparent electro-optical neural interfaces. Adv Funct Mater. 2022, 32, 2106493.
doi: 10.1002/adfm.v32.9 URL |
| 68. |
Lim, C.; Park, C.; Sunwoo, S. H.; Kim, Y. G.; Lee, S.; Han, S. I.; Kim, D.; Kim, J. H.; Kim, D. H.; Hyeon, T. Facile and scalable synthesis of whiskered gold nanosheets for stretchable, conductive, and biocompatible nanocomposites. ACS Nano. 2022, 16, 10431-10442.
doi: 10.1021/acsnano.2c00880 URL |
| 69. | Dong, R.; Wang, L.; Hang, C.; Chen, Z.; Liu, X.; Zhong, L.; Qi, J.; Huang, Y.; Liu, S.; Wang, L.; Lu, Y.; Jiang, X. Printed stretchable liquid metal electrode arrays for in vivo neural recording. Small. 2021, 17, e2006612. |
| 70. |
Guo, R.; Liu, J. Implantable liquid metal-based flexible neural microelectrode array and its application in recovering animal locomotion functions. J Micromech Microeng. 2017, 27, 104002.
doi: 10.1088/1361-6439/aa891c URL |
| 71. | Zhang, X.; Liu, B.; Gao, J.; Lang, Y.; Lv, X.; Deng, Z.; Gui, L.; Liu, J.; Tang, R.; Li, L. Liquid metal-based electrode array for neural signal recording. Bioengineering (Basel). 2023, 10, 578. |
| 72. |
Tang, R.; Zhang, C.; Liu, B.; Jiang, C.; Wang, L.; Zhang, X.; Huang, Q.; Liu, J.; Li, L. Towards an artificial peripheral nerve: Liquid metal-based fluidic cuff electrodes for long-term nerve stimulation and recording. Biosens Bioelectron. 2022, 216, 114600.
doi: 10.1016/j.bios.2022.114600 URL |
| 73. |
Kim, D.; Thissen, P.; Viner, G.; Lee, D. W.; Choi, W.; Chabal, Y. J.; Lee, J. B. Recovery of nonwetting characteristics by surface modification of gallium-based liquid metal droplets using hydrochloric acid vapor. ACS Appl Mater Interfaces. 2013, 5, 179-185.
doi: 10.1021/am302357t URL |
| 74. |
So, J. H.; Koo, H. J.; Dickey, M. D.; Velev, O. D. Ionic current rectification in soft-matter diodes with liquid-metal electrodes. Adv Funct Mater. 2012, 22, 625-631.
doi: 10.1002/adfm.v22.3 URL |
| 75. |
Lee, S. H.; Thunemann, M.; Lee, K.; Cleary, D. R.; Tonsfeldt, K. J.; Oh, H.; Azzazy, F.; Tchoe, Y.; Bourhis, A. M.; Hossain, L.; Ro, Y. G.; Tanaka, A.; Kılıç, K.; Devor, A.; Dayeh, S. A. Scalable thousand channel penetrating microneedle arrays on flex for multimodal and large area coverage brainmachine interfaces. Adv Funct Mater. 2022, 32, 2112045.
doi: 10.1002/adfm.v32.25 URL |
| 76. | Suzuki, I.; Matsuda, N.; Han, X.; Noji, S.; Shibata, M.; Nagafuku, N.; Ishibashi, Y. Large-area field potential imaging having single neuron resolution using 236 880 electrodes CMOS-MEA technology. Adv Sci (Weinh). 2023, 10, e2207732. |
| 77. |
Li, S.; Shi, Q.; Li, Y.; Yang, J.; Chang, T. H.; Jiang, J.; Chen, P. Y. Intercalation of metal ions into Ti3C2Tx MXene electrodes for high-areal-capacitance microsupercapacitors with neutral multivalent electrolytes. Adv Funct Mater. 2020, 30, 2003721.
doi: 10.1002/adfm.v30.40 URL |
| 78. |
Rafieerad, A.; Amiri, A.; Sequiera, G. L.; Yan, W.; Chen, Y.; Polycarpou, A. A.; Dhingra, S. Development of fluorine-free tantalum carbide MXene hybrid structure as a biocompatible material for supercapacitor electrodes. Adv Funct Mater. 2021, 31, 2100015.
doi: 10.1002/adfm.v31.30 URL |
| 79. |
Zhang, Y.; Zhang, L.; Li, C.; Han, J.; Huang, W.; Zhou, J.; Yang, Y. Hydrophilic antifouling 3D porous MXene/holey graphene nanocomposites for electrochemical determination of dopamine. Microchem J. 2022, 181, 107713.
doi: 10.1016/j.microc.2022.107713 URL |
| 80. |
Xu, J.; Shirinkami, H.; Hwang, S.; Jeong, H. S.; Kim, G.; Jun, S. B.; Chun, H. Fast reconfigurable electrode array based on titanium oxide for localized stimulation of cultured neural network. ACS Appl Mater Interfaces. 2023, 15, 19092-19101.
doi: 10.1021/acsami.2c21649 URL |
| 81. | Zhang, F.; Zhang, L.; Xia, J.; Zhao, W.; Dong, S.; Ye, Z.; Pan, G.; Luo, J.; Zhang, S. Multimodal electrocorticogram active electrode array based on zinc oxide-thin film transistors. Adv Sci (Weinh). 2023, 10, e2204467. |
| 82. |
Liu, S.; Liu, L.; Zhao, Y.; Wang, Y.; Wu, Y.; Zhang, X. D.; Ming, D. A high-performance electrode based on van der waals heterostructure for neural recording. Nano Lett. 2022, 22, 4400-4409.
doi: 10.1021/acs.nanolett.2c00848 URL |
| 83. |
Park, D. W.; Brodnick, S. K.; Ness, J. P.; Atry, F.; Krugner-Higby, L.; Sandberg, A.; Mikael, S.; Richner, T. J.; Novello, J.; Kim, H.; Baek, D. H.; Bong, J.; Frye, S. T.; Thongpang, S.; Swanson, K. I.; Lake, W.; Pashaie, R.; Williams, J. C.; Ma, Z. Fabrication and utility of a transparent graphene neural electrode array for electrophysiology, in vivo imaging, and optogenetics. Nat Protoc. 2016, 11, 2201-2222.
doi: 10.1038/nprot.2016.127 |
| 84. | Park, S. Y.; Park, J.; Sim, S. H.; Sung, M. G.; Kim, K. S.; Hong, B. H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv Mater. 2011, 23, H263-267. |
| 85. |
Liu, X.; Xu, Z.; Fu, X.; Liu, Y.; Jia, H.; Yang, Z.; Zhang, J.; Wei, S.; Duan, X. Stable, long-term single-neuronal recording from the rat spinal cord with flexible carbon nanotube fiber electrodes. J Neural Eng. 2022, 19, 056024.
doi: 10.1088/1741-2552/ac9258 |
| 86. |
Yang, H.; Qian, Z.; Wang, J.; Feng, J.; Tang, C.; Wang, L.; Guo, Y.; Liu, Z.; Yang, Y.; Zhang, K.; Chen, P.; Sun, X.; Peng, H. Carbon nanotube array-based flexible multifunctional electrodes to record electrophysiology and ions on the cerebral cortex in real time. Adv Funct Mater. 2022, 32, 2204794.
doi: 10.1002/adfm.v32.38 URL |
| 87. | Xiong, Z.; Huang, W.; Liang, Q.; Cao, Y.; Liu, S.; He, Z.; Zhang, R.; Zhang, B.; Green, R.; Zhang, S.; Li, D. Harnessing the 2D structure-enabled viscoelasticity of graphene-based hydrogel membranes for chronic neural interfacing. Small Methods. 2022, 6, e2200022. |
| 88. |
Yang, X.; Zhu, J.; Qiu, L.; Li, D. Bioinspired effective prevention of restacking in multilayered graphene films: towards the next generation of high-performance supercapacitors. Adv Mater. 2011, 23, 2833-2838.
doi: 10.1002/adma.v23.25 URL |
| 89. |
Xiong, J.; Zhang, B.; Balilonda, A.; Yang, S.; Li, K.; Zhang, Q.; Li, Y.; Wang, H.; Hou, C. Graphene-based implantable neural electrodes for insect flight control. J Mater Chem B. 2022, 10, 4632-4639.
doi: 10.1039/D2TB00906D URL |
| 90. |
Goding, J.; Gilmour, A.; Martens, P.; Poole-Warren, L.; Green, R. Interpenetrating conducting hydrogel materials for neural interfacing electrodes. Adv Healthc Mater. 2017, 6, 1601177.
doi: 10.1002/adhm.v6.9 URL |
| 91. | Tomaskovic-Crook, E.; Zhang, P.; Ahtiainen, A.; Kaisvuo, H.; Lee, C. Y.; Beirne, S.; Aqrawe, Z.; Svirskis, D.; Hyttinen, J.; Wallace, G. G.; Travas-Sejdic, J.; Crook, J. M. Human neural tissues from neural stem cells using conductive biogel and printed polymer microelectrode arrays for 3D electrical stimulation. Adv Healthc Mater. 2019, 8, e1900425. |
| 92. |
Widge, A. S.; Jeffries-El, M.; Cui, X.; Lagenaur, C. F.; Matsuoka, Y. Self-assembled monolayers of polythiophene conductive polymers improve biocompatibility and electrical impedance of neural electrodes. Biosens Bioelectron. 2007, 22, 1723-1732.
doi: 10.1016/j.bios.2006.08.011 URL |
| 93. | Liang, Q.; Shen, Z.; Sun, X.; Yu, D.; Liu, K.; Mugo, S. M.; Chen, W.; Wang, D.; Zhang, Q. Electron conductive and transparent hydrogels for recording brain neural signals and neuromodulation. Adv Mater. 2023, 35, e2211159. |
| 94. |
Xia, X.; Liang, Q.; Sun, X.; Yu, D.; Huang, X.; Mugo, S. M.; Chen, W.; Wang, D.; Zhang, Q. Intrinsically electron conductive, antibacterial, and anti-swelling hydrogels as implantable sensors for bioelectronics. Adv Funct Mater. 2022, 32, 2208024.
doi: 10.1002/adfm.v32.48 URL |
| 95. |
Rinoldi, C.; Ziai, Y.; Zargarian, S. S.; Nakielski, P.; Zembrzycki, K.; Haghighat Bayan, M. A.; Zakrzewska, A. B.; Fiorelli, R.; Lanzi, M.; Kostrzewska-Księżyk, A.; Czajkowski, R.; Kublik, E.; Kaczmarek, L.; Pierini, F. In vivo chronic brain cortex signal recording based on a soft conductive hydrogel biointerface. ACS Appl Mater Interfaces. 2023, 15, 6283-6296.
doi: 10.1021/acsami.2c17025 URL |
| 96. |
Won, C.; Jeong, U. J.; Lee, S.; Lee, M.; Kwon, C.; Cho, S.; Yoon, K.; Lee, S.; Chun, D.; Cho, I. J.; Lee, T. Mechanically tissue-like and highly conductive au nanoparticles embedded elastomeric fiber electrodes of brain-machine interfaces for chronic in vivo brain neural recording. Adv Funct Mater. 2022, 32, 2205145.
doi: 10.1002/adfm.v32.52 URL |
| 97. | Carli, S.; Bianchi, M.; Zucchini, E.; Di Lauro, M.; Prato, M.; Murgia, M.; Fadiga, L.; Biscarini, F. Electrodeposited PEDOT:nafion composite for neural recording and stimulation. Adv Healthc Mater. 2019, 8, e1900765. |
| 98. |
Mandal, H. S.; Knaack, G. L.; Charkhkar, H.; McHail, D. G.; Kastee, J. S.; Dumas, T. C.; Peixoto, N.; Rubinson, J. F.; Pancrazio, J. J. Improving the performance of poly(3,4-ethylenedioxythiophene) for brain-machine interface applications. Acta Biomater. 2014, 10, 2446-2454.
doi: 10.1016/j.actbio.2014.02.029 URL |
| 99. |
Pranti, A. S.; Schander, A.; Bödecker, A.; Lang, W. PEDOT: PSS coating on gold microelectrodes with excellent stability and high charge injection capacity for chronic neural interfaces. Sens Actuators B Chem. 2018, 275, 382-393.
doi: 10.1016/j.snb.2018.08.007 URL |
| 100. |
Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A. M.; Tok, J. B.; Bao, Z. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat Biomed Eng. 2019, 3, 58-68.
doi: 10.1038/s41551-018-0335-6 |
| 101. |
Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat Commun. 2019, 10, 1043.
doi: 10.1038/s41467-019-09003-5 |
| 102. |
Feig, V. R.; Tran, H.; Lee, M.; Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat Commun. 2018, 9, 2740.
doi: 10.1038/s41467-018-05222-4 |
| 103. |
Li, T. L.; Liu, Y.; Forro, C.; Yang, X.; Beker, L.; Bao, Z.; Cui, B.; Pașca, S. P. Stretchable mesh microelectronics for the biointegration and stimulation of human neural organoids. Biomaterials. 2022, 290, 121825.
doi: 10.1016/j.biomaterials.2022.121825 URL |
| 104. |
Wang, S.; Guan, S.; Wang, J.; Liu, H.; Liu, T.; Ma, X.; Cui, Z. Fabrication and characterization of conductive poly (3,4-ethylenedioxythiophene) doped with hyaluronic acid/poly (l-lactic acid) composite film for biomedical application. J Biosci Bioeng. 2017, 123, 116-125.
doi: 10.1016/j.jbiosc.2016.07.010 URL |
| 105. |
Ohm, Y.; Pan, C.; Ford, M. J.; Huang, X.; Liao, J.; Majidi, C. An electrically conductive silver-polyacrylamide-alginate hydrogel composite for soft electronics. Nat Electron. 2021, 4, 185-192.
doi: 10.1038/s41928-021-00545-5 |
| 106. |
Alizadeh, R.; Zarrintaj, P.; Kamrava, S. K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutiérrez, T. J.; Saeb, M. R. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr Polym. 2019, 224, 115161.
doi: 10.1016/j.carbpol.2019.115161 URL |
| 107. |
Mili, B.; Das, K.; Kumar, A.; Saxena, A. C.; Singh, P.; Ghosh, S.; Bag, S. Preparation of NGF encapsulated chitosan nanoparticles and its evaluation on neuronal differentiation potentiality of canine mesenchymal stem cells. J Mater Sci Mater Med. 2017, 29, 4.
doi: 10.1007/s10856-017-6008-2 URL |
| 108. |
Qasim, S. B.; Zafar, M. S.; Najeeb, S.; Khurshid, Z.; Shah, A. H.; Husain, S.; Rehman, I. U. Electrospinning of chitosan-based solutions for tissue engineering and regenerative medicine. Int J Mol Sci. 2018, 19, 407.
doi: 10.3390/ijms19020407 URL |
| 109. |
Aznar-Cervantes, S.; Pagán, A.; Martínez, J. G.; Bernabeu-Esclapez, A.; Otero, T. F.; Meseguer-Olmo, L.; Paredes, J. I.; Cenis, J. L. Electrospun silk fibroin scaffolds coated with reduced graphene promote neurite outgrowth of PC-12 cells under electrical stimulation. Mater Sci Eng C Mater Biol Appl. 2017, 79, 315-325.
doi: 10.1016/j.msec.2017.05.055 URL |
| 110. | Cui, Y.; Zhang, F.; Chen, G.; Yao, L.; Zhang, N.; Liu, Z.; Li, Q.; Zhang, F.; Cui, Z.; Zhang, K.; Li, P.; Cheng, Y.; Zhang, S.; Chen, X. A stretchable and transparent electrode based on PEGylated silk fibroin for in vivo dual-modal neural-vascular activity probing. Adv Mater. 2021, 33, e2100221. |
| 111. |
Ding, J.; Chen, Z.; Liu, X.; Tian, Y.; Jiang, J.; Qiao, Z.; Zhang, Y.; Xiao, Z.; Wei, D.; Sun, J.; Luo, F.; Zhou, L.; Fan, H. A mechanically adaptive hydrogel neural interface based on silk fibroin for high-efficiency neural activity recording. Mater Horiz. 2022, 9, 2215-2225.
doi: 10.1039/D2MH00533F URL |
| 112. | Cho, Y.; Borgens, R. B. The effect of an electrically conductive carbon nanotube/collagen composite on neurite outgrowth of PC12 cells. J Biomed Mater Res A. 2010, 95, 510-517. |
| 113. |
Liu, X.; Yue, Z.; Higgins, M. J.; Wallace, G. G. Conducting polymers with immobilised fibrillar collagen for enhanced neural interfacing. Biomaterials. 2011, 32, 7309-7317.
doi: 10.1016/j.biomaterials.2011.06.047 URL |
| 114. |
Yue, Z.; Liu, X.; Molino, P. J.; Wallace, G. G. Bio-functionalisation of polydimethylsiloxane with hyaluronic acid and hyaluronic acid--collagen conjugate for neural interfacing. Biomaterials. 2011, 32, 4714-4724.
doi: 10.1016/j.biomaterials.2011.03.032 URL |
| 115. |
Dhawan, V.; Cui, X. T. Carbohydrate based biomaterials for neural interface applications. J Mater Chem B. 2022, 10, 4714-4740.
doi: 10.1039/D2TB00584K URL |
| 116. |
Zhou, Y.; Gu, C.; Liang, J.; Zhang, B.; Yang, H.; Zhou, Z.; Li, M.; Sun, L.; Tao, T. H.; Wei, X. A silk-based self-adaptive flexible opto-electro neural probe. Microsyst Nanoeng. 2022, 8, 118.
doi: 10.1038/s41378-022-00461-4 |
| 117. |
Yang, J.; Du, M.; Wang, L.; Li, S.; Wang, G.; Yang, X.; Zhang, L.; Fang, Y.; Zheng, W.; Yang, G.; Jiang, X. Bacterial cellulose as a supersoft neural interfacing substrate. ACS Appl Mater Interfaces. 2018, 10, 33049-33059.
doi: 10.1021/acsami.8b12083 URL |
| 118. |
Potter-Baker, K. A.; Stewart, W. G.; Tomaszewski, W. H.; Wong, C. T.; Meador, W. D.; Ziats, N. P.; Capadona, J. R. Implications of chronic daily anti-oxidant administration on the inflammatory response to intracortical microelectrodes. J Neural Eng. 2015, 12, 046002.
doi: 10.1088/1741-2560/12/4/046002 URL |
| 119. |
Golabchi, A.; Woeppel, K. M.; Li, X.; Lagenaur, C. F.; Cui, X. T. Neuroadhesive protein coating improves the chronic performance of neuroelectronics in mouse brain. Biosens Bioelectron. 2020, 155, 112096.
doi: 10.1016/j.bios.2020.112096 URL |
| 120. |
Azemi, E.; Lagenaur, C. F.; Cui, X. T. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials. 2011, 32, 681-692.
doi: 10.1016/j.biomaterials.2010.09.033 URL |
| 121. |
Oakes, R. S.; Polei, M. D.; Skousen, J. L.; Tresco, P. A. An astrocyte derived extracellular matrix coating reduces astrogliosis surrounding chronically implanted microelectrode arrays in rat cortex. Biomaterials. 2018, 154, 1-11.
doi: 10.1016/j.biomaterials.2017.10.001 URL |
| 122. |
Vitale, F.; Shen, W.; Driscoll, N.; Burrell, J. C.; Richardson, A. G.; Adewole, O.; Murphy, B.; Ananthakrishnan, A.; Oh, H.; Wang, T.; Lucas, T. H.; Cullen, D. K.; Allen, M. G.; Litt, B. Biomimetic extracellular matrix coatings improve the chronic biocompatibility of microfabricated subdural microelectrode arrays. PLoS One. 2018, 13, e0206137.
doi: 10.1371/journal.pone.0206137 URL |
| 123. | Ramesh, V.; Stratmann, N.; Schaufler, V.; Angelov, S. D.; Nordhorn, I. D.; Heissler, H. E.; Martínez-Hincapié, R.; Čolić, V.; Rehbock, C.; Schwabe, K.; Karst, U.; Krauss, J. K.; Barcikowski, S. Mechanical stability of nano-coatings on clinically applicable electrodes, generated by electrophoretic deposition. Adv Healthc Mater. 2022, 11, e2102637. |
| 124. | Ramesh, V.; Rehbock, C.; Giera, B.; Karnes, J. J.; Forien, J. B.; Angelov, S. D.; Schwabe, K.; Krauss, J. K.; Barcikowski, S. Comparing direct and pulsed-direct current electrophoretic deposition on neural electrodes: deposition mechanism and functional influence. Langmuir. 2021. doi: 10.1021/acs.langmuir.1c01081. |
| 125. |
Angelov, S. D.; Koenen, S.; Jakobi, J.; Heissler, H. E.; Alam, M.; Schwabe, K.; Barcikowski, S.; Krauss, J. K. Electrophoretic deposition of ligand-free platinum nanoparticles on neural electrodes affects their impedance in vitro and in vivo with no negative effect on reactive gliosis. J Nanobiotechnology. 2016, 14, 3.
doi: 10.1186/s12951-015-0154-9 URL |
| 126. |
Yang, D.; Tian, G.; Liang, C.; Yang, Z.; Zhao, Q.; Chen, J.; Ma, C.; Jiang, Y.; An, N.; Liu, Y.; Qi, D. Double-microcrack coupling stretchable neural electrode for electrophysiological communication. Adv Funct Mater. 2023, 33, 2300412.
doi: 10.1002/adfm.v33.37 URL |
| 127. |
Nguyen, T. K.; Barton, M.; Ashok, A.; Truong, T. A.; Yadav, S.; Leitch, M.; Nguyen, T. V.; Kashaninejad, N.; Dinh, T.; Hold, L.; Yamauchi, Y.; Nguyen, N. T.; Phan, H. P. Wide bandgap semiconductor nanomembranes as a long-term biointerface for flexible, implanted neuromodulator. Proc Natl Acad Sci U S A. 2022, 119, e2203287119.
doi: 10.1073/pnas.2203287119 URL |
| 128. |
Dong, M.; Coleman, H. A.; Tonta, M. A.; Xiong, Z.; Li, D.; Thomas, S.; Liu, M.; Fallon, J. B.; Parkington, H. C.; Forsythe, J. S. Rapid electrophoretic deposition of biocompatible graphene coatings for high-performance recording neural electrodes. Nanoscale. 2022, 14, 15845-15858.
doi: 10.1039/D2NR04421H URL |
| 129. |
Xiao, G.; Song, Y.; Zhang, Y.; Xing, Y.; Xu, S.; Lu, Z.; Wang, M.; Cai, X. Cellular-scale microelectrode arrays to monitor movement-related neuron activities in the epileptic hippocampus of awake mice. IEEE Trans Biomed Eng. 2021, 68, 19-25.
doi: 10.1109/TBME.10 URL |
| 130. |
Huang, W. C.; Hung, C. H.; Lin, Y. W.; Zheng, Y. C.; Lei, W. L.; Lu, H. E. Electrically copolymerized polydopamine melanin/poly(3,4-ethylenedioxythiophene) applied for bioactive multimodal neural interfaces with induced pluripotent stem cell-derived neurons. ACS Biomater Sci Eng. 2022, 8, 4807-4818.
doi: 10.1021/acsbiomaterials.2c00822 URL |
| 131. |
Saunier, V.; Flahaut, E.; Blatché, M. C.; Bergaud, C.; Maziz, A. Carbon nanofiber-PEDOT composite films as novel microelectrode for neural interfaces and biosensing. Biosens Bioelectron. 2020, 165, 112413.
doi: 10.1016/j.bios.2020.112413 URL |
| 132. |
Yang, X.; Pei, W.; Wei, C.; Yang, X.; Zhang, H.; Wang, Y.; Yuan, M.; Gui, Q.; Liu, Y.; Wang, Y.; Chen, H. Chemical polymerization of conducting polymer poly(3,4-ethylenedioxythiophene) onto neural microelectrodes. Sens Actuators A Phys. 2023, 349, 114022.
doi: 10.1016/j.sna.2022.114022 URL |
| 133. | Lim, T.; Kim, M.; Akbarian, A.; Kim, J.; Tresco, P. A.; Zhang, H. Conductive polymer enabled biostable liquid metal electrodes for bioelectronic applications. Adv Healthc Mater. 2022, 11, e2102382. |
| 134. | Ganji, M.; Hossain, L.; Tanaka, A.; Thunemann, M.; Halgren, E.; Gilja, V.; Devor, A.; Dayeh, S. A. Monolithic and scalable Au nanorod substrates improve PEDOT-metal adhesion and stability in neural electrodes. Adv Healthc Mater. 2018, 7, e1800923. |
| 135. |
Wei, B.; Liu, J.; Ouyang, L.; Kuo, C. C.; Martin, D. C. Significant enhancement of PEDOT thin film adhesion to inorganic solid substrates with EDOT-acid. ACS Appl Mater Interfaces. 2015, 7, 15388-15394.
doi: 10.1021/acsami.5b03350 URL |
| 136. |
Istif, E.; Mantione, D.; Vallan, L.; Hadziioannou, G.; Brochon, C.; Cloutet, E.; Pavlopoulou, E. Thiophene-based aldehyde derivatives for functionalizable and adhesive semiconducting polymers. ACS Appl Mater Interfaces. 2020, 12, 8695-8703.
doi: 10.1021/acsami.9b21058 URL |
| 137. |
Tian, F.; Yu, J.; Wang, W.; Zhao, D.; Cao, J.; Zhao, Q.; Wang, F.; Yang, H.; Wu, Z.; Xu, J.; Lu, B. Design of adhesive conducting PEDOT-MeOH:PSS/PDA neural interface via electropolymerization for ultrasmall implantable neural microelectrodes. J Colloid Interface Sci. 2023, 638, 339-348.
doi: 10.1016/j.jcis.2023.01.146 URL |
| 138. |
Desroches, P. E.; Silva, S. M.; Gietman, S. W.; Quigley, A. F.; Kapsa, R. M. I.; Moulton, S. E.; Greene, G. W. Lubricin (PRG4) antiadhesive coatings mitigate electrochemical impedance instabilities in polypyrrole bionic electrodes exposed to fouling fluids. ACS Appl Bio Mater. 2020, 3, 8032-8039.
doi: 10.1021/acsabm.0c01109 URL |
| 139. |
Wellens, J.; Deschaume, O.; Putzeys, T.; Eyley, S.; Thielemans, W.; Verhaert, N.; Bartic, C. Sulfobetaine-based ultrathin coatings as effective antifouling layers for implantable neuroprosthetic devices. Biosens Bioelectron. 2023, 226, 115121.
doi: 10.1016/j.bios.2023.115121 URL |
| 140. |
Jeong, J. O.; Kim, S.; Park, J.; Lee, S.; Park, J. S.; Lim, Y. M.; Lee, J. Y. Biomimetic nonbiofouling polypyrrole electrodes grafted with zwitterionic polymer using gamma rays. J Mater Chem B. 2020, 8, 7225-7232.
doi: 10.1039/C9TB02087J URL |
| 141. | Lee, Y.; Shin, H.; Lee, D.; Choi, S.; Cho, I. J.; Seo, J. A lubricated nonimmunogenic neural probe for acute insertion trauma minimization and long-term signal recording. Adv Sci (Weinh). 2021, 8, e2100231. |
| 142. | Jain, V.; Forssell, M.; Tansel, D. Z.; Goswami, C.; Fedder, G. K.; Grover, P.; Chamanzar, M. Focused epicranial brain stimulation by spatial sculpting of pulsed electric fields using high density electrode arrays. Adv Sci (Weinh). 2023, 10, e2207251. |
| 143. |
Chen, Z.; Liu, X.; Ding, J.; Tian, Y.; Zhang, Y.; Wei, D.; Sun, J.; Luo, F.; Zhou, L.; Fan, H. Tissue-like electrophysiological electrode interface construction by multiple crosslinked polysaccharide-based hydrogel. Carbohydr Polym. 2022, 296, 119923.
doi: 10.1016/j.carbpol.2022.119923 URL |
| 144. |
Fabbro, A.; Prato, M.; Ballerini, L. Carbon nanotubes in neuroregeneration and repair. Adv Drug Deliv Rev. 2013, 65, 2034-2044.
doi: 10.1016/j.addr.2013.07.002 URL |
| 145. |
Ramer, L. M.; Ramer, M. S.; Bradbury, E. J. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 2014, 13, 1241-1256.
doi: 10.1016/S1474-4422(14)70144-9 URL |
| 146. | Ye, L.; Ji, H.; Liu, J.; Tu, C. H.; Kappl, M.; Koynov, K.; Vogt, J.; Butt, H. J. Carbon nanotube-hydrogel composites facilitate neuronal differentiation while maintaining homeostasis of network activity. Adv Mater. 2021, 33, e2102981. |
| 147. |
Tian, G.; Yang, D.; Chen, C.; Duan, X.; Kim, D. H.; Chen, H. Simultaneous presentation of dexamethasone and nerve growth factor via layered carbon nanotubes and polypyrrole to interface neural cells. ACS Biomater Sci Eng. 2023, 9, 5015-5027.
doi: 10.1021/acsbiomaterials.3c00593 URL |
| 148. |
Wei, W.; Hao, M.; Zhou, K.; Wang, Y.; Lu, Q.; Zhang, H.; Wu, Y.; Zhang, T.; Liu, Y. In situ multimodal transparent electrophysiological hydrogel for in vivo miniature two-photon neuroimaging and electrocorticogram analysis. Acta Biomater. 2022, 152, 86-99.
doi: 10.1016/j.actbio.2022.08.053 URL |
| 149. | Lee, J.; Jeong, H.; Kim, J.; Seo, J. M. Investigation of neural electrode fabrication process on Polycarbonate substrate. Annu Int Conf IEEE Eng Med Biol Soc. 2022, 2022, 3089-3092. |
| 150. |
Baek, C.; Kim, J.; Lee, Y.; Seo, J. M. Fabrication and evaluation of cyclic olefin copolymer based implantable neural electrode. IEEE Trans Biomed Eng. 2020, 67, 2542-2551.
doi: 10.1109/TBME.10 URL |
| 151. |
Altuna, A.; Menendez de la Prida, L.; Bellistri, E.; Gabriel, G.; Guimerá, A.; Berganzo, J.; Villa, R.; Fernández, L. J. SU-8 based microprobes with integrated planar electrodes for enhanced neural depth recording. Biosens Bioelectron. 2012, 37, 1-5.
doi: 10.1016/j.bios.2012.03.039 URL |
| 152. |
Ware, T.; Simon, D.; Arreaga-Salas, D. E.; Reeder, J.; Rennaker, R.; Keefer, E. W.; Voit, W. Fabrication of responsive, softening neural interfaces. Adv Funct Mater. 2012, 22, 3470-3479.
doi: 10.1002/adfm.v22.16 URL |
| 153. |
Castagnola, E.; Ansaldo, A.; Fadiga, L.; Ricci, D. Chemical vapour deposited carbon nanotube coated microelectrodes for intracortical neural recording. Phys Status Solidi B. 2010, 247, 2703-2707.
doi: 10.1002/pssb.v247.11/12 URL |
| 154. |
Choi, D. S.; Fung, A. O.; Moon, H.; Villareal, G.; Chen, Y.; Ho, D.; Presser, N.; Stupian, G.; Leung, M. Detection of neural signals with vertically grown single platinum nanowire-nanobud. J Nanosci Nanotechnol. 2009, 9, 6483-6486.
doi: 10.1166/jnn.2009.1462 URL |
| 155. | Paik, S. J.; Cho, D. D. Development of recording microelectrodes with low surface impedance for neural chip applications. J Korean Phys Soc. 2002, 41, 1046-1049. |
| 156. |
Niederhoffer, T.; Vanhoestenberghe, A.; Lancashire, H. T. Methods of poly(3,4)-ethylenedioxithiophene (PEDOT) electrodeposition on metal electrodes for neural stimulation and recording. J Neural Eng. 2023, 20, 011002.
doi: 10.1088/1741-2552/acb084 |
| 157. | Wang, Y.; Graham, E. S.; Unsworth, C. P. Superior galvanostatic electrochemical deposition of platinum nanograss provides high performance planar microelectrodes forin vitroneural recording. J Neural Eng. 2021, 18, 0460d0468. |
| 158. |
Zhang, C.; Driver, N.; Tian, Q.; Jiang, W.; Liu, H. Electrochemical deposition of conductive polymers onto magnesium microwires for neural electrode applications. J Biomed Mater Res A. 2018, 106, 1887-1895.
doi: 10.1002/jbm.a.v106.7 URL |
| 159. |
Abidian, M. R.; Martin, D. C. Multifunctional nanobiomaterials for neural interfaces. Adv Funct Mater. 2009, 19, 573-585.
doi: 10.1002/adfm.v19:4 URL |
| 160. |
Heo, D. N.; Kim, H. J.; Lee, Y. J.; Heo, M.; Lee, S. J.; Lee, D.; Do, S. H.; Lee, S. H.; Kwon, I. K. Flexible and highly biocompatible nanofiber-based electrodes for neural surface interfacing. ACS Nano. 2017, 11, 2961-2971.
doi: 10.1021/acsnano.6b08390 URL |
| 161. | Jenkins, P. M.; Laughter, M. R.; Lee, D. J.; Lee, Y. M.; Freed, C. R.; Park, D. A nerve guidance conduit with topographical and biochemical cues: potential application using human neural stem cells. Nanoscale Res Lett. 2015, 10, 972. |
| 162. |
James, C. D.; Davis, R.; Meyer, M.; Turner, A.; Turner, S.; Withers, G.; Kam, L.; Banker, G.; Craighead, H.; Isaacson, M.; Turner, J.; Shain, W. Aligned microcontact printing of micrometer-scale poly-L-lysine structures for controlled growth of cultured neurons on planar microelectrode arrays. IEEE Trans Biomed Eng. 2000, 47, 17-21.
doi: 10.1109/10.817614 URL |
| 163. | Beom Jun, S.; Hynd, M.; Dowell-Mesfin, N.; Smith, K.; Turner, J.; Shain, W.; June Kim, S. Synaptic connectivity of a low density patterned neuronal network produced on the poly-L-lysine stamped microelectrode array. Conf Proc IEEE Eng Med Biol Soc. 2005, 2005, 7604-7607. |
| 164. |
Mehenti, N. Z.; Tsien, G. S.; Leng, T.; Fishman, H. A.; Bent, S. F. A model retinal interface based on directed neuronal growth for single cell stimulation. Biomed Microdevices. 2006, 8, 141-150.
doi: 10.1007/s10544-006-7709-3 URL |
| 165. |
Seo, K. J.; Hill, M.; Ryu, J.; Chiang, C. H.; Rachinskiy, I.; Qiang, Y.; Jang, D.; Trumpis, M.; Wang, C.; Viventi, J.; Fang, H. A soft, high-density neuroelectronic array. Npj Flex Electron. 2023, 7, 40.
doi: 10.1038/s41528-023-00271-2 |
| 166. | Roh, H.; Yoon, Y. J.; Park, J. S.; Kang, D. H.; Kwak, S. M.; Lee, B. C.; Im, M. Fabrication of high-density out-of-plane microneedle arrays with various heights and diverse cross-sectional shapes. Nanomicro Lett. 2021, 14, 24. |
| 167. |
Ando, D.; Teshima, T. F.; Zurita, F.; Peng, H.; Ogura, K.; Kondo, K.; Weiß, L.; Hirano-Iwata, A.; Becherer, M.; Alexander, J.; Wolfrum, B. Filtration-processed biomass nanofiber electrodes for flexible bioelectronics. J Nanobiotechnology. 2022, 20, 491.
doi: 10.1186/s12951-022-01684-3 |
| 168. |
Mailley, S. C.; Hyland, M.; Mailley, P.; McLaughlin, J. M.; McAdams, E. T. Electrochemical and structural characterizations of electrodeposited iridium oxide thin-film electrodes applied to neurostimulating electrical signal. Mater Sci Eng C. 2002, 21, 167-175.
doi: 10.1016/S0928-4931(02)00098-X URL |
| 169. |
Stöver, T.; Paasche, G.; Lenarz, T.; Ripken, T.; Breitenfeld, P.; Lubatschowski, H.; Fabian, T. Development of a drug delivery device: using the femtosecond laser to modify cochlear implant electrodes. Cochlear Implants Int. 2007, 8, 38-52.
doi: 10.1179/cim.2007.8.1.38 URL |
| 170. |
Henle, C.; Raab, M.; Cordeiro, J. G.; Doostkam, S.; Schulze-Bonhage, A.; Stieglitz, T.; Rickert, J. First long term in vivo study on subdurally implanted micro-ECoG electrodes, manufactured with a novel laser technology. Biomed Microdevices. 2011, 13, 59-68.
doi: 10.1007/s10544-010-9471-9 URL |
| 171. |
Li, L.; Jiang, C.; Li, L. Hierarchical platinum-iridium neural electrodes structured by femtosecond laser for superwicking interface and superior charge storage capacity. Bio-des Manuf. 2022, 5, 163-173.
doi: 10.1007/s42242-021-00160-5 |
| 172. |
Yang, Q.; Hu, Z.; Seo, M. H.; Xu, Y.; Yan, Y.; Hsu, Y. H.; Berkovich, J.; Lee, K.; Liu, T. L.; McDonald, S.; Nie, H.; Oh, H.; Wu, M.; Kim, J. T.; Miller, S. A.; Jia, Y.; Butun, S.; Bai, W.; Guo, H.; Choi, J.; Banks, A.; Ray, W. Z.; Kozorovitskiy, Y.; Becker, M. L.; Pet, M. A.; MacEwan, M. R.; Chang, J. K.; Wang, H.; Huang, Y.; Rogers, J. A. High-speed, scanned laser structuring of multi-layered eco/bioresorbable materials for advanced electronic systems. Nat Commun. 2022, 13, 6518.
doi: 10.1038/s41467-022-34173-0 |
| 173. |
Amini, S.; Seche, W.; May, N.; Choi, H.; Tavousi, P.; Shahbazmohamadi, S. Femtosecond laser hierarchical surface restructuring for next generation neural interfacing electrodes and microelectrode arrays. Sci Rep. 2022, 12, 13966.
doi: 10.1038/s41598-022-18161-4 |
| 174. |
Won, D.; Kim, J.; Choi, J.; Kim, H.; Han, S.; Ha, I.; Bang, J.; Kim, K. K.; Lee, Y.; Kim, T. S.; Park, J. H.; Kim, C. Y.; Ko, S. H. Digital selective transformation and patterning of highly conductive hydrogel bioelectronics by laser-induced phase separation. Sci Adv. 2022, 8, eabo3209.
doi: 10.1126/sciadv.abo3209 URL |
| 175. | Pant, M.; Singh, R.; Negi, P.; Tiwari, K.; Singh, Y. A comprehensive review on carbon nano-tube synthesis using chemical vapor deposition. Mater Today Proc. 2021, 46, 11250-11253. |
| 176. |
Ansaldo, A.; Castagnola, E.; Maggiolini, E.; Fadiga, L.; Ricci, D. Superior electrochemical performance of carbon nanotubes directly grown on sharp microelectrodes. ACS Nano. 2011, 5, 2206-2214.
doi: 10.1021/nn103445d URL |
| 177. |
Lee, M.; Lee, S.; Kim, J.; Lim, J.; Lee, J.; Masri, S.; Bao, S.; Yang, S.; Ahn, J. H.; Yang, S. Graphene-electrode array for brain map remodeling of the cortical surface. NPG Asia Mater. 2021, 13, 65.
doi: 10.1038/s41427-021-00334-8 |
| 178. |
Bakhshaee Babaroud, N.; Palmar, M.; Velea, A. I.; Coletti, C.; Weingärtner, S.; Vos, F.; Serdijn, W. A.; Vollebregt, S.; Giagka, V. Multilayer CVD graphene electrodes using a transfer-free process for the next generation of optically transparent and MRI-compatible neural interfaces. Microsyst Nanoeng. 2022, 8, 107.
doi: 10.1038/s41378-022-00430-x |
| 179. |
Lee, J. Y.; Bashur, C. A.; Goldstein, A. S.; Schmidt, C. E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009, 30, 4325-4335.
doi: 10.1016/j.biomaterials.2009.04.042 URL |
| 180. |
Mousavi, H.; Ferrari, L. M.; Whiteley, A.; Ismailova, E. Kinetics and physicochemical characteristics of electrodeposited PEDOT:PSS thin film growth. Adv Electron Mater. 2023, 9, 2201282.
doi: 10.1002/aelm.v9.9 URL |
| 181. |
Yang, M.; Yang, T.; Deng, H.; Wang, J.; Ning, S.; Li, X.; Ren, X.; Su, Y.; Zang, J.; Li, X.; Luo, Z. Poly(5-nitroindole) thin film as conductive and adhesive interfacial layer for robust neural interface. Adv Funct Mater. 2021, 31, 2105857.
doi: 10.1002/adfm.v31.49 URL |
| 182. |
Skoog, S. A.; Kumar, G.; Narayan, R. J.; Goering, P. L. Biological responses to immobilized microscale and nanoscale surface topographies. Pharmacol Ther. 2018, 182, 33-55.
doi: 10.1016/j.pharmthera.2017.07.009 URL |
| 183. |
Newman, P.; Galenano Niño, J. L.; Graney, P.; Razal, J. M.; Minett, A. I.; Ribas, J.; Ovalle-Robles, R.; Biro, M.; Zreiqat, H. Relationship between nanotopographical alignment and stem cell fate with live imaging and shape analysis. Sci Rep. 2016, 6, 37909.
doi: 10.1038/srep37909 |
| 184. |
Zijl, S.; Vasilevich, A. S.; Viswanathan, P.; Helling, A. L.; Beijer, N. R. M.; Walko, G.; Chiappini, C.; de Boer, J.; Watt, F. M. Micro-scaled topographies direct differentiation of human epidermal stem cells. Acta Biomater. 2019, 84, 133-145.
doi: 10.1016/j.actbio.2018.12.003 URL |
| 185. |
Simitzi, C.; Ranella, A.; Stratakis, E. Controlling the morphology and outgrowth of nerve and neuroglial cells: The effect of surface topography. Acta Biomater. 2017, 51, 21-52.
doi: 10.1016/j.actbio.2017.01.023 URL |
| 186. | Luo, J.; Walker, M.; Xiao, Y.; Donnelly, H.; Dalby, M. J.; Salmeron-Sanchez, M. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix - a review. Bioact Mater. 2022, 15, 145-159. |
| 187. |
Geiger, B.; Spatz, J. P.; Bershadsky, A. D. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009, 10, 21-33.
doi: 10.1038/nrm2593 |
| 188. |
Lord, M. S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today. 2010, 5, 66-78.
doi: 10.1016/j.nantod.2010.01.001 URL |
| 189. |
Kim, M. H.; Park, M.; Kang, K.; Choi, I. S. Neurons on nanometric topographies: insights into neuronal behaviors in vitro. Biomater Sci. 2014, 2, 148-155.
doi: 10.1039/C3BM60255A URL |
| 190. |
Arora, S.; Lin, S.; Cheung, C.; Yim, E. K. F.; Toh, Y. C. Topography elicits distinct phenotypes and functions in human primary and stem cell derived endothelial cells. Biomaterials. 2020, 234, 119747.
doi: 10.1016/j.biomaterials.2019.119747 URL |
| 191. |
Curtis, A.; Wilkinson, C. Topographical control of cells. Biomaterials. 1997, 18, 1573-1583.
doi: 10.1016/S0142-9612(97)00144-0 URL |
| 192. |
Nowduri, B.; Schulte, S.; Decker, D.; Schäfer, K. H.; Saumer, M. Biomimetic nanostructures fabricated by nanoimprint lithography for improved cell-coupling. Adv Funct Mater. 2020, 30, 2004227.
doi: 10.1002/adfm.v30.45 URL |
| 193. | Tringides, C. M.; Boulingre, M.; Khalil, A.; Lungjangwa, T.; Jaenisch, R.; Mooney, D. J. Tunable conductive hydrogel scaffolds for neural cell differentiation. Adv Healthc Mater. 2023, 12, e2202221. |
| 194. | Wang, J.; Wang, H.; Mo, X.; Wang, H. Reduced graphene oxide-encapsulated microfiber patterns enable controllable formation of neuronal-like networks. Adv Mater. 2020, 32, e2004555. |
| [1] | Long Bai, Peiran Song, Jiacan Su. Bioactive elements manipulate bone regeneration [J]. Biomaterials Translational, 2023, 4(4): 248-269. |
| [2] | Qiao Sun, Yicun Li, Ping Luo, Hong He. Animal models for testing biomaterials in periodontal regeneration [J]. Biomaterials Translational, 2023, 4(3): 142-150. |
| [3] | Jingyu Fan, Elizabeth Pung, Yuan Lin, Qian Wang. Recent development of hydrogen sulfide-releasing biomaterials as novel therapies:a narrative review [J]. Biomaterials Translational, 2022, 3(4): 250-263. |
| [4] | Yiqiang Hu, Yuan Xiong, Ranyang Tao, Hang Xue, Lang Chen, Ze Lin, Adriana C. Panayi, Bobin Mi, Guohui Liu. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing [J]. Biomaterials Translational, 2022, 3(3): 188-200. |
| [5] | Shuqin Cao, Quan Yuan. An update of nanotopographical surfaces in modulating stem cell fate: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 55-64. |
| [6] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [7] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [8] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [9] | Yu Lu, Subodh Deshmukh, Ian Jones, Yu-Lung Chiu. Biodegradable magnesium alloys for orthopaedic applications [J]. Biomaterials Translational, 2021, 2(3): 214-235. |
| [10] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [11] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [12] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [13] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||