Biomaterials Translational ›› 2023, Vol. 4 ›› Issue (3): 142-150.doi: 10.12336/biomatertransl.2023.03.003
• REVIEW • Previous Articles Next Articles
Qiao Sun1, Yicun Li3, Ping Luo1, Hong He1,2,*( )
)
Received:2023-02-25
Revised:2023-06-02
Accepted:2023-09-04
Online:2023-09-28
Published:2023-09-28
Contact:
*Hong He,
Sun, Q.; Li, Y.; Luo, P.; He, H. Animal models for testing biomaterials in periodontal regeneration. Biomater Transl. 2023, 4(3), 142-150.
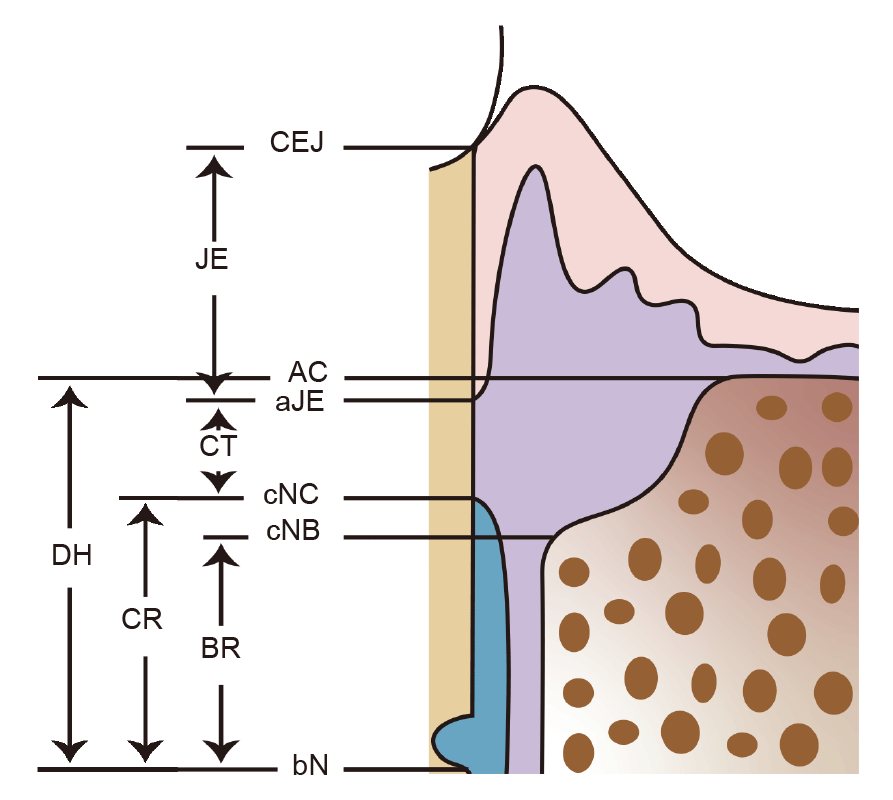
Figure 3. Schematic drawing representing measurement points for histometric parameters. AC: alveolar crest; aJE: apical extension of the junctional epithelium; bN: base of the reference notch; BR: bone regeneration; CEJ: cemento–enamel junction; cNB: coronal extension of newly formed bone; cNC: coronal extension of newly formed cementum; CR: cementum regeneration; CT: connective tissue attachment; DH: defect height; JE: junctional epithelial attachment.
| Animal species | Characteristic | Type of model | Site of defect | Size of defect | Longest period for evaluation |
|---|---|---|---|---|---|
| Mouse | Low–cost; Immunodeficient mice and gene–manipulated mice are available; Regenerative procedures are difficult to conduct due to the narrow oral cavity. | Chronic periodontal defect model | 15 days | ||
| Heterotopic transplantation model | 8 weeks | ||||
| Rat | Well established as a model for periodontitis; Physiological remodelling of periodontal tissues due to continuous eruption of molars; More likely to form horizontal bone loss due to the narrow interdental spaces. | Acute periodontal defect model | Fenestration defect | 2 × 5 × 1.2 mm3 | 3 weeks |
| Intrabony defect | 2 × 2 × 1.7 mm3 | 12 weeks | |||
| Other defect models | Mandibular angle defect | 5 mm in diameter | 6 weeks | ||
| Calvarial defect | 8 mm in diameter | 12 months | |||
| Rabbit | Periodontitis can be induced; Fenestration defects may be replaced rather than regenerated due to the continuous eruption of their teeth. | Other defect models | Calvarial defect | 8 mm in diameter | 8 weeks |
| Dog | Size and anatomy of periodontal tissues are comparable to humans; Naturally developed periodontitis; The daily care and postoperative maintenance are more complex than rodents. | Acute periodontal defect model | Intrabony defect | 5 × 5 × 5 mm3 or 5 × 5 × 4 mm3 or 4 × 4 × 4 mm3 | 24 weeks |
| Supra–alveolar defect | 5 mm | 8 weeks | |||
| Furcation defect | 5 × 5 × 3 mm3 | 120 days | |||
| Recession–type defect | 6 × 5 mm2 | 8 weeks | |||
| Interproximal periodontal defect | 5 × 3 mm2 | 8 weeks | |||
| Miniature pig | Similarities to humans in periodontal anatomy and the presence of naturally occurring periodontitis. | Acute periodontal defect model | Recession–type defect | 5 × 4 mm2 | 3 months |
| Nonhuman primate | The anatomy of teeth and periodontium in nonhuman primates closely resembles that of humans; An excellent animal model for evaluating regenerative procedures; Ethical concerns, requirements of specialised facilities and high costs. | Acute/chronic periodontal defect model | 7–9 × 2–3 mm2 or 3 × 4 × 5 mm3 | 5 months |
Table 1. Animal models for periodontal regeneration
| Animal species | Characteristic | Type of model | Site of defect | Size of defect | Longest period for evaluation |
|---|---|---|---|---|---|
| Mouse | Low–cost; Immunodeficient mice and gene–manipulated mice are available; Regenerative procedures are difficult to conduct due to the narrow oral cavity. | Chronic periodontal defect model | 15 days | ||
| Heterotopic transplantation model | 8 weeks | ||||
| Rat | Well established as a model for periodontitis; Physiological remodelling of periodontal tissues due to continuous eruption of molars; More likely to form horizontal bone loss due to the narrow interdental spaces. | Acute periodontal defect model | Fenestration defect | 2 × 5 × 1.2 mm3 | 3 weeks |
| Intrabony defect | 2 × 2 × 1.7 mm3 | 12 weeks | |||
| Other defect models | Mandibular angle defect | 5 mm in diameter | 6 weeks | ||
| Calvarial defect | 8 mm in diameter | 12 months | |||
| Rabbit | Periodontitis can be induced; Fenestration defects may be replaced rather than regenerated due to the continuous eruption of their teeth. | Other defect models | Calvarial defect | 8 mm in diameter | 8 weeks |
| Dog | Size and anatomy of periodontal tissues are comparable to humans; Naturally developed periodontitis; The daily care and postoperative maintenance are more complex than rodents. | Acute periodontal defect model | Intrabony defect | 5 × 5 × 5 mm3 or 5 × 5 × 4 mm3 or 4 × 4 × 4 mm3 | 24 weeks |
| Supra–alveolar defect | 5 mm | 8 weeks | |||
| Furcation defect | 5 × 5 × 3 mm3 | 120 days | |||
| Recession–type defect | 6 × 5 mm2 | 8 weeks | |||
| Interproximal periodontal defect | 5 × 3 mm2 | 8 weeks | |||
| Miniature pig | Similarities to humans in periodontal anatomy and the presence of naturally occurring periodontitis. | Acute periodontal defect model | Recession–type defect | 5 × 4 mm2 | 3 months |
| Nonhuman primate | The anatomy of teeth and periodontium in nonhuman primates closely resembles that of humans; An excellent animal model for evaluating regenerative procedures; Ethical concerns, requirements of specialised facilities and high costs. | Acute/chronic periodontal defect model | 7–9 × 2–3 mm2 or 3 × 4 × 5 mm3 | 5 months |
| 1. | Nazir, M. A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017, 11, 72-80. |
| 2. |
Kwon, T.; Lamster, I. B.; Levin, L. Current concepts in the management of periodontitis. Int Dent J. 2021, 71, 462-476.
doi: 10.1111/idj.12630 URL |
| 3. |
Melcher, A. H. On the repair potential of periodontal tissues. J Periodontol. 1976, 47, 256-260.
doi: 10.1902/jop.1976.47.5.256 URL |
| 4. |
Nyman, S.; Gottlow, J.; Karring, T.; Lindhe, J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. 1982, 9, 257-265.
doi: 10.1111/cpe.1982.9.issue-3 URL |
| 5. |
Villar, C. C.; Cochran, D. L. Regeneration of periodontal tissues: guided tissue regeneration. Dent Clin North Am. 2010, 54, 73-92.
doi: 10.1016/j.cden.2009.08.011 URL |
| 6. |
Xu, X. Y.; Li, X.; Wang, J.; He, X. T.; Sun, H. H.; Chen, F. M. Concise review: periodontal tissue regeneration using stem cells: strategies and translational considerations. Stem Cells Transl Med. 2019, 8, 392-403.
doi: 10.1002/sctm.18-0181 URL |
| 7. |
Struillou, X.; Boutigny, H.; Soueidan, A.; Layrolle, P. Experimental animal models in periodontology: a review. Open Dent J. 2010, 4, 37-47.
doi: 10.2174/1874210601004010037 URL |
| 8. |
Kantarci, A.; Hasturk, H.; Van Dyke, T. E. Animal models for periodontal regeneration and peri-implant responses. Periodontol 2000. 2015, 68, 66-82.
doi: 10.1111/prd.2015.68.issue-1 URL |
| 9. | Nuñez, J.; Sanchez, N.; Vignoletti, F.; Sanz-Martin, I.; Caffesse, R.; Santamaria, S.; Garcia-Sanz, J. A.; Sanz, M. Cell therapy with allogenic canine periodontal ligament-derived cells in periodontal regeneration of critical size defects. J Clin Periodontol. 2018, 45, 453-461. |
| 10. |
França-Grohmann, I. L.; Sangiorgio, J. P. M.; Bueno, M. R.; Casarin, R. C. V.; Silvério Ruiz, K. G.; Nociti, F. H., Jr.; Casati, M. Z.; Sallum, E. A. Treatment of dehiscence-type defects with collagen matrix and/or enamel matrix derivative: histomorphometric study in minipigs. J Periodontol. 2020, 91, 967-974.
doi: 10.1002/jper.v91.7 URL |
| 11. |
Jimbo, R.; Singer, J.; Tovar, N.; Marin, C.; Neiva, R.; Bonfante, E. A.; Janal, M. N.; Contamin, H.; Coelho, P. G. Regeneration of the cementum and periodontal ligament using local BDNF delivery in class II furcation defects. J Biomed Mater Res B Appl Biomater. 2018, 106, 1611-1617.
doi: 10.1002/jbm.v106.4 URL |
| 12. |
Farag, A.; Hashimi, S. M.; Vaquette, C.; Bartold, P. M.; Hutmacher, D. W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J Clin Periodontol. 2018, 45, 586-596.
doi: 10.1111/jcpe.2018.45.issue-5 URL |
| 13. |
Hasani-Sadrabadi, M. M.; Sarrion, P.; Nakatsuka, N.; Young, T. D.; Taghdiri, N.; Ansari, S.; Aghaloo, T.; Li, S.; Khademhosseini, A.; Weiss, P. S.; Moshaverinia, A. Hierarchically patterned polydopamine-containing membranes for periodontal tissue engineering. ACS Nano. 2019, 13, 3830-3838.
doi: 10.1021/acsnano.8b09623 URL |
| 14. |
Batool, F.; Morand, D. N.; Thomas, L.; Bugueno, I. M.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a novel electrospun polycaprolactone scaffold functionalized with ibuprofen for periodontal regeneration: an in vitro and in vivo study. Materials (Basel). 2018, 11, 580.
doi: 10.3390/ma11040580 URL |
| 15. | Khuda, F.; Baharin, B.; Anuar, N. N. M.; Satimin, B. S. F.; Nasruddin, N. S. Effective Modalities of Periodontitis Induction in Rat Model. J Vet Dent. 2023, 8987564231178459. |
| 16. |
Heijl, L.; Wennström, J.; Lindhe, J.; Socransky, S. S. Periodontal disease in gnotobiotic rats. J Periodontal Res. 1980, 15, 405-419.
doi: 10.1111/jre.1980.15.issue-4 URL |
| 17. |
Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials. 2019, 206, 115-132.
doi: 10.1016/j.biomaterials.2019.03.039 URL |
| 18. |
Shang, F.; Liu, S.; Ming, L.; Tian, R.; Jin, F.; Ding, Y.; Zhang, Y.; Zhang, H.; Deng, Z.; Jin, Y. Human umbilical cord MSCs as new cell sources for promoting periodontal regeneration in inflammatory periodontal defect. Theranostics. 2017, 7, 4370-4382.
doi: 10.7150/thno.19888 URL |
| 19. |
Dan, H.; Vaquette, C.; Fisher, A. G.; Hamlet, S. M.; Xiao, Y.; Hutmacher, D. W.; Ivanovski, S. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials. 2014, 35, 113-122.
doi: 10.1016/j.biomaterials.2013.09.074 URL |
| 20. |
Zhang, Y.; Wei, L.; Wu, C.; Miron, R. J. Periodontal regeneration using strontium-loaded mesoporous bioactive glass scaffolds in osteoporotic rats. PLoS One. 2014, 9, e104527.
doi: 10.1371/journal.pone.0104527 URL |
| 21. |
Zheng, B.; Jiang, J.; Chen, Y.; Lin, M.; Du, Z.; Xiao, Y.; Luo, K.; Yan, F. Leptin overexpression in bone marrow stromal cells promotes periodontal regeneration in a rat model of osteoporosis. J Periodontol. 2017, 88, 808-818.
doi: 10.1902/jop.2017.170042 URL |
| 22. |
Oortgiesen, D. A.; Walboomers, X. F.; Bronckers, A. L.; Meijer, G. J.; Jansen, J. A. Periodontal regeneration using an injectable bone cement combined with BMP-2 or FGF-2. J Tissue Eng Regen Med. 2014, 8, 202-209.
doi: 10.1002/term.v8.3 URL |
| 23. |
Cai, X.; Yang, F.; Yan, X.; Yang, W.; Yu, N.; Oortgiesen, D. A.; Wang, Y.; Jansen, J. A.; Walboomers, X. F. Influence of bone marrow-derived mesenchymal stem cells pre-implantation differentiation approach on periodontal regeneration in vivo. J Clin Periodontol. 2015, 42, 380-389.
doi: 10.1111/jcpe.2015.42.issue-4 URL |
| 24. |
El-Sayed, B.; Davies, R. P. W.; El-Zehery, R. R.; Ibrahim, F. M.; Grawish, M. E.; Kirkham, J.; El-Gendy, R. An in-vivo intraoral defect model for assessing the use of p(11)-4 self-assembling peptide in periodontal regeneration. Front Bioeng Biotechnol. 2020, 8, 559494.
doi: 10.3389/fbioe.2020.559494 URL |
| 25. |
Liu, X.; He, X.; Jin, D.; Wu, S.; Wang, H.; Yin, M.; Aldalbahi, A.; El-Newehy, M.; Mo, X.; Wu, J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020, 108, 207-222.
doi: 10.1016/j.actbio.2020.03.044 URL |
| 26. |
Xu, X.; Zhou, Y.; Zheng, K.; Li, X.; Li, L.; Xu, Y. 3D polycaprolactone/gelatin-oriented electrospun scaffolds promote periodontal regeneration. ACS Appl Mater Interfaces. 2022, 14, 46145-46160.
doi: 10.1021/acsami.2c03705 URL |
| 27. |
Nemcovsky, C. E.; Zahavi, S.; Moses, O.; Kebudi, E.; Artzi, Z.; Beny, L.; Weinreb, M. Effect of enamel matrix protein derivative on healing of surgical supra-infrabony periodontal defects in the rat molar: a histomorphometric study. J Periodontol. 2006, 77, 996-1002.
doi: 10.1902/jop.2006.050317 URL |
| 28. |
Oortgiesen, D. A.; Meijer, G. J.; Bronckers, A. L.; Walboomers, X. F.; Jansen, J. A. Regeneration of the periodontium using enamel matrix derivative in combination with an injectable bone cement. Clin Oral Investig. 2013, 17, 411-421.
doi: 10.1007/s00784-012-0743-z URL |
| 29. | Babo, P. S.; Cai, X.; Plachokova, A. S.; Reis, R. L.; Jansen, J.; Gomes, M. E.; Walboomers, X. F. Evaluation of a platelet lysate bilayered system for periodontal regeneration in a rat intrabony three-wall periodontal defect. J Tissue Eng Regen Med. 2018, 12, e1277-e1288. |
| 30. |
Hasturk, H.; Jones, V. L.; Andry, C.; Kantarci, A. 1-Tetradecanol complex reduces progression of Porphyromonas gingivalis-induced experimental periodontitis in rabbits. J Periodontol. 2007, 78, 924-932.
doi: 10.1902/jop.2007.060293 URL |
| 31. |
Oortgiesen, D. A.; Meijer, G. J.; Bronckers, A. L.; Walboomers, X. F.; Jansen, J. A. Fenestration defects in the rabbit jaw: an inadequate model for studying periodontal regeneration. Tissue Eng Part C Methods. 2010, 16, 133-140.
doi: 10.1089/ten.tec.2009.0191 URL |
| 32. |
Schmitt, J. M.; Buck, D. C.; Joh, S. P.; Lynch, S. E.; Hollinger, J. O. Comparison of porous bone mineral and biologically active glass in critical-sized defects. J Periodontol. 1997, 68, 1043-1053.
doi: 10.1902/jop.1997.68.11.1043 URL |
| 33. |
Lee, J. Y.; Park, J. Y.; Hong, I. P.; Jeon, S. H.; Cha, J. K.; Paik, J. W.; Choi, S. H. 3D-printed barrier membrane using mixture of polycaprolactone and beta-tricalcium phosphate for regeneration of rabbit calvarial defects. Materials (Basel). 2021, 14, 3280.
doi: 10.3390/ma14123280 URL |
| 34. |
Hamp, S. E.; Hamp, M.; Olsson, S. E.; Lindberg, R.; Schauman, P. Radiography of spontaneous periodontitis in dogs. J Periodontal Res. 1997, 32, 589-597.
doi: 10.1111/jre.1997.32.issue-7 URL |
| 35. |
Haney, J. M.; Zimmerman, G. J.; Wikesjö, U. M. Periodontal repair in dogs: evaluation of the natural disease model. J Clin Periodontol. 1995, 22, 208-213.
doi: 10.1111/j.1600-051X.1995.tb00136.x URL |
| 36. |
Kim, C. S.; Choi, S. H.; Chai, J. K.; Cho, K. S.; Moon, I. S.; Wikesjö, U. M.; Kim, C. K. Periodontal repair in surgically created intrabony defects in dogs: influence of the number of bone walls on healing response. J Periodontol. 2004, 75, 229-235.
doi: 10.1902/jop.2004.75.2.229 URL |
| 37. |
Matsuse, K.; Hashimoto, Y.; Kakinoki, S.; Yamaoka, T.; Morita, S. Periodontal regeneration induced by porous alpha-tricalcium phosphate with immobilized basic fibroblast growth factor in a canine model of 2-wall periodontal defects. Med Mol Morphol. 2018, 51, 48-56.
doi: 10.1007/s00795-017-0172-9 URL |
| 38. |
Imber, J. C.; Bosshardt, D. D.; Stähli, A.; Saulacic, N.; Deschner, J.; Sculean, A. Pre-clinical evaluation of the effect of a volume-stable collagen matrix on periodontal regeneration in two-wall intrabony defects. J Clin Periodontol. 2021, 48, 560-569.
doi: 10.1111/jcpe.v48.4 URL |
| 39. |
Shirakata, Y.; Taniyama, K.; Yoshimoto, T.; Miyamoto, M.; Takeuchi, N.; Matsuyama, T.; Noguchi, K. Regenerative effect of basic fibroblast growth factor on periodontal healing in two-wall intrabony defects in dogs. J Clin Periodontol. 2010, 37, 374-381.
doi: 10.1111/cpe.2010.37.issue-4 URL |
| 40. |
Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009, 30, 2716-2723.
doi: 10.1016/j.biomaterials.2009.01.032 URL |
| 41. |
Kim, C. K.; Kim, H. Y.; Chai, J. K.; Cho, K. S.; Moon, I. S.; Choi, S. H.; Sottosanti, J. S.; Wikesjö, U. M. Effect of a calcium sulfate implant with calcium sulfate barrier on periodontal healing in 3-wall intrabony defects in dogs. J Periodontol. 1998, 69, 982-988.
doi: 10.1902/jop.1998.69.9.982 URL |
| 42. |
Shirakata, Y.; Setoguchi, F.; Sena, K.; Nakamura, T.; Imafuji, T.; Shinohara, Y.; Iwata, M.; Noguchi, K. Comparison of periodontal wound healing/regeneration by recombinant human fibroblast growth factor-2 combined with β-tricalcium phosphate, carbonate apatite, or deproteinized bovine bone mineral in a canine one-wall intra-bony defect model. J Clin Periodontol. 2022, 49, 599-608.
doi: 10.1111/jcpe.v49.6 URL |
| 43. |
Choi, S. H.; Kim, C. K.; Cho, K. S.; Huh, J. S.; Sorensen, R. G.; Wozney, J. M.; Wikesjö, U. M. Effect of recombinant human bone morphogenetic protein-2/absorbable collagen sponge (rhBMP-2/ACS) on healing in 3-wall intrabony defects in dogs. J Periodontol. 2002, 73, 63-72.
doi: 10.1902/jop.2002.73.1.63 URL |
| 44. |
Jung, U. W.; Chang, Y. Y.; Um, Y. J.; Kim, C. S.; Cho, K. S.; Choi, S. H. Interproximal periodontal defect model in dogs: a pilot study. Oral Dis. 2011, 17, 26-32.
doi: 10.1111/odi.2010.17.issue-1 URL |
| 45. |
Wikesjö, U. M.; Selvig, K. A.; Zimmerman, G.; Nilvéus, R. Periodontal repair in dogs: healing in experimentally created chronic periodontal defects. J Periodontol. 1991, 62, 258-263.
doi: 10.1902/jop.1991.62.4.258 URL |
| 46. |
Wei, L.; Teng, F.; Deng, L.; Liu, G.; Luan, M.; Jiang, J.; Liu, Z.; Liu, Y. Periodontal regeneration using bone morphogenetic protein 2 incorporated biomimetic calcium phosphate in conjunction with barrier membrane: a pre-clinical study in dogs. J Clin Periodontol. 2019, 46, 1254-1263.
doi: 10.1111/jcpe.v46.12 URL |
| 47. |
Garrett, S.; Gantes, B.; Zimmerman, G.; Egelberg, J. Treatment of mandibular class III periodontal furcation defects. Coronally positioned flaps with and without expanded polytetrafluoroethylene membranes. J Periodontol. 1994, 65, 592-597.
doi: 10.1902/jop.1994.65.6.592 URL |
| 48. |
Carlo Reis, E. C.; Borges, A. P.; Araújo, M. V.; Mendes, V. C.; Guan, L.; Davies, J. E. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials. 2011, 32, 9244-9253.
doi: 10.1016/j.biomaterials.2011.08.040 URL |
| 49. |
Chantarawaratit, P.; Sangvanich, P.; Banlunara, W.; Soontornvipart, K.; Thunyakitpisal, P. Acemannan sponges stimulate alveolar bone, cementum and periodontal ligament regeneration in a canine class II furcation defect model. J Periodontal Res. 2014, 49, 164-178.
doi: 10.1111/jre.2014.49.issue-2 URL |
| 50. |
Suaid, F. A.; Macedo, G. O.; Novaes, A. B.; Borges, G. J.; Souza, S. L.; Taba, M.; Palioto, D. B.; Grisi, M. F. The bone formation capabilities of the anorganic bone matrix-synthetic cell-binding peptide 15 grafts in an animal periodontal model: a histologic and histomorphometric study in dogs. J Periodontol. 2010, 81, 594-603.
doi: 10.1902/jop.2010.090486 URL |
| 51. |
Shujaa Addin, A.; Akizuki, T.; Hoshi, S.; Matsuura, T.; Ikawa, T.; Fukuba, S.; Matsui, M.; Tabata, Y.; Izumi, Y. Biodegradable gelatin/beta-tricalcium phosphate sponges incorporating recombinant human fibroblast growth factor-2 for treatment of recession-type defects: A split-mouth study in dogs. J Periodontal Res. 2017, 52, 863-871.
doi: 10.1111/jre.2017.52.issue-5 URL |
| 52. |
Wang, S.; Liu, Y.; Fang, D.; Shi, S. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007, 13, 530-537.
doi: 10.1111/odi.2007.13.issue-6 URL |
| 53. |
Schou, S.; Holmstrup, P.; Kornman, K. S. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. 1993, 64, 497-508.
doi: 10.1902/jop.1993.64.6.497 URL |
| 54. |
Caton, J.; Mota, L.; Gandini, L.; Laskaris, B. Non-human primate models for testing the efficacy and safety of periodontal regeneration procedures. J Periodontol. 1994, 65, 1143-1150.
doi: 10.1902/jop.1994.65.12.1143 URL |
| 55. |
Caton, J. G.; Kowalski, C. J. Primate model for testing periodontal treatment procedures: II. Production of contralaterally similar lesions. J Periodontol. 1976, 47, 506-510.
doi: 10.1902/jop.1976.47.9.506 URL |
| 56. |
Yamashita, M.; Lazarov, M.; Jones, A. A.; Mealey, B. L.; Mellonig, J. T.; Cochran, D. L. Periodontal regeneration using an anabolic peptide with two carriers in baboons. J Periodontol. 2010, 81, 727-736.
doi: 10.1902/jop.2010.090224 URL |
| 57. |
Emerton, K. B.; Drapeau, S. J.; Prasad, H.; Rohrer, M.; Roffe, P.; Hopper, K.; Schoolfield, J.; Jones, A.; Cochran, D. L. Regeneration of periodontal tissues in non-human primates with rhGDF-5 and beta-tricalcium phosphate. J Dent Res. 2011, 90, 1416-1421.
doi: 10.1177/0022034511423665 URL |
| 58. |
Wang, B.; Mastrogiacomo, S.; Yang, F.; Shao, J.; Ong, M. M. A.; Chanchareonsook, N.; Jansen, J. A.; Walboomers, X. F.; Yu, N. Application of BMP-bone cement and FGF-gel on periodontal tissue regeneration in nonhuman primates. Tissue Eng Part C Methods. 2019, 25, 748-756.
doi: 10.1089/ten.tec.2019.0160 URL |
| 59. |
Zellin, G.; Gritli-Linde, A.; Linde, A. Healing of mandibular defects with different biodegradable and non-biodegradable membranes: an experimental study in rats. Biomaterials. 1995, 16, 601-609.
doi: 10.1016/0142-9612(95)93857-A URL |
| 60. |
Spicer, P. P.; Kretlow, J. D.; Young, S.; Jansen, J. A.; Kasper, F. K.; Mikos, A. G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc. 2012, 7, 1918-1929.
doi: 10.1038/nprot.2012.113 |
| 61. |
Polimeni, G.; Koo, K. T.; Pringle, G. A.; Agelan, A.; Safadi, F. F.; Wikesjö, U. M. Histopathological observations of a polylactic acid-based device intended for guided bone/tissue regeneration. Clin Implant Dent Relat Res. 2008, 10, 99-105.
doi: 10.1111/j.1708-8208.2007.00067.x URL |
| 62. |
An, Y. Z.; Heo, Y. K.; Lee, J. S.; Jung, U. W.; Choi, S. H. Dehydrothermally cross-linked collagen membrane with a bone graft improves bone regeneration in a rat calvarial defect model. Materials (Basel). 2017, 10, 927.
doi: 10.3390/ma10080927 URL |
| 63. |
Jang, J. W.; Lee, J. S.; Jung, U. W.; Kim, C. S.; Cho, K. S. In vivo evaluation of commercially available gel-type polyethylene glycol membrane for carrier of recombinant human bone morphogenetic protein-2. J Oral Maxillofac Surg. 2017, 75, 297.e1-297.e13.
doi: 10.1016/S0278-2391(17)30595-5 URL |
| 64. |
Jung, U. W.; Choi, S. Y.; Pang, E. K.; Kim, C. S.; Choi, S. H.; Cho, K. S. The effect of varying the particle size of beta tricalcium phosphate carrier of recombinant human bone morphogenetic protein-4 on bone formation in rat calvarial defects. J Periodontol. 2006, 77, 765-772.
doi: 10.1902/jop.2006.050268 URL |
| 65. |
Jung, U. W.; Song, K. Y.; Kim, C. S.; Lee, Y. K.; Cho, K. S.; Kim, C. K.; Choi, S. H. Effects of a chitosan membrane coated with polylactic and polyglycolic acid on bone regeneration in a rat calvarial defect. Biomed Mater. 2007, 2, S101-105.
doi: 10.1088/1748-6041/2/3/S03 URL |
| 66. |
Pang, E. K.; Im, S. U.; Kim, C. S.; Choi, S. H.; Chai, J. K.; Kim, C. K.; Han, S. B.; Cho, K. S. Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J Periodontol. 2004, 75, 1364-1370.
doi: 10.1902/jop.2004.75.10.1364 URL |
| 67. |
Park, J. C.; So, S. S.; Jung, I. H.; Yun, J. H.; Choi, S. H.; Cho, K. S.; Kim, C. S. Induction of bone formation by Escherichia coli-expressed recombinant human bone morphogenetic protein-2 using block-type macroporous biphasic calcium phosphate in orthotopic and ectopic rat models. J Periodontal Res. 2011, 46, 682-690.
doi: 10.1111/jre.2011.46.issue-6 URL |
| 68. |
You, H.; Lee, E. U.; Kim, Y. K.; Kim, B. C.; Park, J. Y.; Lim, H. C.; Lee, J. S.; Noh, I.; Jung, U. W.; Choi, S. H. Biocompatibility and resorption pattern of newly developed hyaluronic acid hydrogel reinforced three-layer poly (lactide-co-glycolide) membrane: histologic observation in rabbit calvarial defect model. Biomater Res. 2014, 18, 12.
doi: 10.1186/2055-7124-18-12 |
| 69. |
Pae, H. C.; Kang, J. H.; Cha, J. K.; Lee, J. S.; Paik, J. W.; Jung, U. W.; Choi, S. H. Bone regeneration using three-dimensional hexahedron channel structured BCP block in rabbit calvarial defects. J Biomed Mater Res B Appl Biomater. 2019, 107, 2254-2262.
doi: 10.1002/jbm.b.v107.7 URL |
| 70. | Teng, S. H.; Lee, E. J.; Wang, P.; Shin, D. S.; Kim, H. E. Three-layered membranes of collagen/hydroxyapatite and chitosan for guided bone regeneration. J Biomed Mater Res B Appl Biomater. 2008, 87, 132-138. |
| 71. |
Choi, J. Y.; Jung, U. W.; Kim, C. S.; Eom, T. K.; Kang, E. J.; Cho, K. S.; Kim, C. K.; Choi, S. H. The effects of newly formed synthetic peptide on bone regeneration in rat calvarial defects. J Periodontal Implant Sci. 2010, 40, 11-18.
doi: 10.5051/jpis.2010.40.1.11 URL |
| 72. | Zhao, B.; Chen, J.; Zhao, L.; Deng, J.; Li, Q. A simvastatin-releasing scaffold with periodontal ligament stem cell sheets for periodontal regeneration. J Appl Biomater Funct Mater. 2020, 18, 2280800019900094. |
| 73. |
Wang, Z. S.; Feng, Z. H.; Wu, G. F.; Bai, S. Z.; Dong, Y.; Chen, F. M.; Zhao, Y. M. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci Rep. 2016, 6, 28126.
doi: 10.1038/srep28126 |
| 74. |
Liao, Y.; Li, H.; Shu, R.; Chen, H.; Zhao, L.; Song, Z.; Zhou, W. Mesoporous hydroxyapatite/chitosan loaded with recombinant-human amelogenin could enhance antibacterial effect and promote periodontal regeneration. Front Cell Infect Microbiol. 2020, 10, 180.
doi: 10.3389/fcimb.2020.00180 URL |
| 75. |
Varoni, E. M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-based trilayer scaffold for multitissue periodontal regeneration. J Dent Res. 2018, 97, 303-311.
doi: 10.1177/0022034517736255 URL |
| 76. |
Yang, H.; Gao, L. N.; An, Y.; Hu, C. H.; Jin, F.; Zhou, J.; Jin, Y.; Chen, F. M. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials. 2013, 34, 7033-7047.
doi: 10.1016/j.biomaterials.2013.05.025 URL |
| 77. |
Gao, L. N.; An, Y.; Lei, M.; Li, B.; Yang, H.; Lu, H.; Chen, F. M.; Jin, Y. The effect of the coumarin-like derivative osthole on the osteogenic properties of human periodontal ligament and jaw bone marrow mesenchymal stem cell sheets. Biomaterials. 2013, 34, 9937-9951.
doi: 10.1016/j.biomaterials.2013.09.017 URL |
| 78. |
Park, J. C.; Oh, S. Y.; Lee, J. S.; Park, S. Y.; Choi, E. Y.; Cho, K. S.; Kim, C. S. In vivo bone formation by human alveolar-bone-derived mesenchymal stem cells obtained during implant osteotomy using biphasic calcium phosphate ceramics or Bio-Oss as carriers. J Biomed Mater Res B Appl Biomater. 2016, 104, 515-524.
doi: 10.1002/jbm.b.v104.3 URL |
| 79. | Gao, H.; Li, B.; Zhao, L.; Jin, Y. Influence of nanotopography on periodontal ligament stem cell functions and cell sheet based periodontal regeneration. Int J Nanomedicine. 2015, 10, 4009-4027. |
| 80. |
Kim, Y. T.; Park, J. C.; Choi, S. H.; Cho, K. S.; Im, G. I.; Kim, B. S.; Kim, C. S. The dynamic healing profile of human periodontal ligament stem cells: histological and immunohistochemical analysis using an ectopic transplantation model. J Periodontal Res. 2012, 47, 514-524.
doi: 10.1111/jre.2012.47.issue-4 URL |
| [1] | Jingyu Fan, Elizabeth Pung, Yuan Lin, Qian Wang. Recent development of hydrogen sulfide-releasing biomaterials as novel therapies:a narrative review [J]. Biomaterials Translational, 2022, 3(4): 250-263. |
| [2] | Yi Wang, Yangyang Chen, Yulong Wei. Osteoarthritis animal models for biomaterial-assisted osteochondral regeneration [J]. Biomaterials Translational, 2022, 3(4): 264-279. |
| [3] | Yiqiang Hu, Yuan Xiong, Ranyang Tao, Hang Xue, Lang Chen, Ze Lin, Adriana C. Panayi, Bobin Mi, Guohui Liu. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing [J]. Biomaterials Translational, 2022, 3(3): 188-200. |
| [4] | Shuqin Cao, Quan Yuan. An update of nanotopographical surfaces in modulating stem cell fate: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 55-64. |
| [5] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [6] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [7] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [8] | Ge Yan, Yuqi Liu, Minghui Xie, Jiawei Shi, Weihua Qiao, Nianguo Dong. Experimental and computational models for tissue-engineered heart valves: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 361-375. |
| [9] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [10] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [11] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [12] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [13] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||