Biomaterials Translational ›› 2022, Vol. 3 ›› Issue (4): 264-279.doi: 10.12336/biomatertransl.2022.04.006
• REVIEW • Previous Articles Next Articles
Yi Wang1, Yangyang Chen2, Yulong Wei2,*( )
)
Received:2022-11-11
Revised:2022-11-26
Accepted:2022-12-10
Online:2022-12-29
Published:2022-12-28
Contact:
Yulong Wei
E-mail:yulongwei@hust.edu.cn
About author:Yulong Wei,yulongwei@hust.edu.cn.#Author Equally.
Wang, Y.; Chen, Y.; Wei, Y. Osteoarthritis animal models for biomaterial-assisted osteochondral regeneration. Biomater Transl. 2022, 3(4), 264-279.
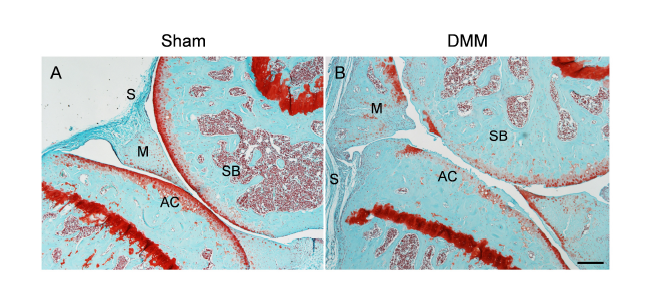
Figure 2. Safranin O and fast green staining of wild–type mouse knee joints at the medial site at 3 months post–sham (A) or post–DMM (B) surgery. DMM surgery was performed by transecting the medial meniscotibial ligament of the knee joint. Sham surgery serves as a control with the meniscotibial ligament intact. Scale bar: 200 μm. AC: articular cartilage; DMM: destabilisation of the medial meniscus; M: meniscus; S: synovium; SB: subchondral bone.
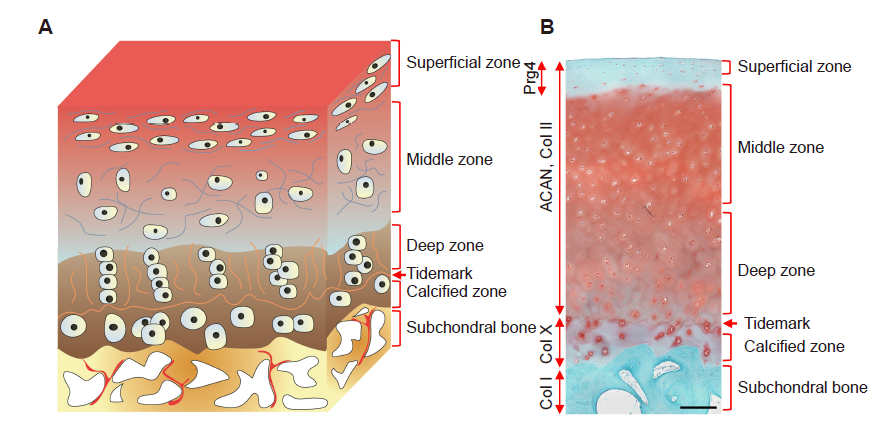
Figure 3. A schematic diagram and representative histological image of an osteochondral unit. (A) The zonal structure of the osteochondral unit. (B) Safranin O and fast green staining of a healthy full–thickness osteochondral unit from an adult human. Scale bar: 200 μm. ACAN: aggrecan; Col I: type I collagen; Col II: type II collagen; Col X: type X collagen; Prg4: proteoglycan 4.
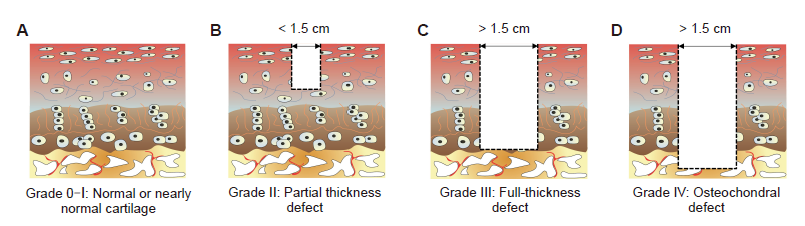
Figure 5. Outerbridge arthroscopic grading system of articular cartilage defects. (A) Grade 0: normal cartilage; Grade I: nearly normal cartilage. (B) Grade II: partial thickness defect. (C) Grade III: full-thickness defect. (D) Grade IV: osteochondral defect.
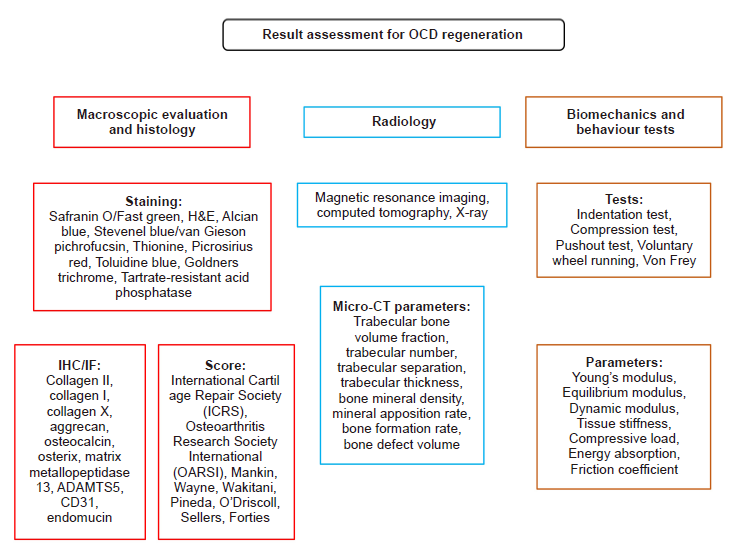
Figure 6. Summary of the evaluation methods for OCD regeneration. ADAMTS5: a disintegrin and metalloproteinase with thrombospondin motif 5; CT: computed tomography; H&E: haematoxylin–eosin; IF: immunofluorescence; IHC: immunohistochemistry; OCD: osteochondral defect.
| 1. |
Vina, E. R.; Kwoh, C. K. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018, 30, 160-167.
doi: 10.1097/BOR.0000000000000479 URL |
| 2. |
Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C. L.; Laslett, L. L.; Jones, G.; Cicuttini, F.; Osborne, R.; Vos, T.; Buchbinder, R.; Woolf, A.; March, L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014, 73, 1323-1330.
doi: 10.1136/annrheumdis-2013-204763 URL |
| 3. |
Cope, P. J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis Cartilage. 2019, 27, 230-239.
doi: 10.1016/j.joca.2018.09.016 URL |
| 4. |
Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013, 21, 1145-1153.
doi: 10.1016/j.joca.2013.03.018 URL |
| 5. |
Kuyinu, E. L.; Narayanan, G.; Nair, L. S.; Laurencin, C. T. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016, 11, 19.
doi: 10.1186/s13018-016-0346-5 URL |
| 6. | Meng, X.; Ziadlou, R.; Grad, S.; Alini, M.; Wen, C.; Lai, Y.; Qin, L.; Zhao, Y.; Wang, X. Animal models of osteochondral defect for testing biomaterials. Biochem Res Int. 2020, 2020, 9659412. |
| 7. |
Li, X.; Ding, J.; Wang, J.; Zhuang, X.; Chen, X. Biomimetic biphasic scaffolds for osteochondral defect repair. Regen Biomater. 2015, 2, 221-228.
doi: 10.1093/rb/rbv015 URL |
| 8. |
Matthews, G. L. Disease modification: promising targets and impediments to success. Rheum Dis Clin North Am. 2013, 39, 177-187.
doi: 10.1016/j.rdc.2012.10.006 URL |
| 9. | Haviv, B.; Bronak, S.; Thein, R. The complexity of pain around the knee in patients with osteoarthritis. Isr Med Assoc J. 2013, 15, 178-181. |
| 10. |
Seo, S. S.; Kim, C. W.; Jung, D. W. Management of focal chondral lesion in the knee joint. Knee Surg Relat Res. 2011, 23, 185-196.
doi: 10.5792/ksrr.2011.23.4.185 URL |
| 11. |
Meng, X.; Grad, S.; Wen, C.; Lai, Y.; Alini, M.; Qin, L.; Wang, X. An impaired healing model of osteochondral defect in papain-induced arthritis. J Orthop Translat. 2021, 26, 101-110.
doi: 10.1016/j.jot.2020.07.005 URL |
| 12. |
Deng, C.; Chang, J.; Wu, C. Bioactive scaffolds for osteochondral regeneration. J Orthop Translat. 2019, 17, 15-25.
doi: 10.1016/j.jot.2018.11.006 URL |
| 13. |
Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T. D.; Greenwald, R.; Hochberg, M.; Howell, D.; Kaplan, D.; Koopman, W.; Longley III, S.; Mankin, H.; McShane, D. J.; Medsger, T.; Meenan, R.; Mikkelsen, W.; Moskowitz, R.; Murphy, W.; Rothschild, B.; Segal, M.; Sokoloff, L.; Wolfe, F. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986, 29, 1039-1049.
doi: 10.1002/art.1780290816 URL |
| 14. | Altman, R. D. Criteria for classification of clinical osteoarthritis. J Rheumatol Suppl. 1991, 27, 10-12. |
| 15. |
Lambova, S. N.; Müller-Ladner, U. Osteoarthritis - current insights in pathogenesis, diagnosis and treatment. Curr Rheumatol Rev. 2018, 14, 91-97.
doi: 10.2174/157339711402180706144757 URL |
| 16. |
Mow, V. C.; Ratcliffe, A.; Poole, A. R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992, 13, 67-97.
doi: 10.1016/0142-9612(92)90001-5 URL |
| 17. |
Armiento, A. R.; Stoddart, M. J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018, 65, 1-20.
doi: 10.1016/j.actbio.2017.11.021 URL |
| 18. |
Pearle, A. D.; Warren, R. F.; Rodeo, S. A. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005, 24, 1-12.
doi: 10.1016/j.csm.2004.08.007 URL |
| 19. |
Lyons, T. J.; Stoddart, R. W.; McClure, S. F.; McClure, J. The tidemark of the chondro-osseous junction of the normal human knee joint. J Mol Histol. 2005, 36, 207-215.
doi: 10.1007/s10735-005-3283-x URL |
| 20. |
Zhang, Y.; Wang, F.; Tan, H.; Chen, G.; Guo, L.; Yang, L. Analysis of the mineral composition of the human calcified cartilage zone. Int J Med Sci. 2012, 9, 353-360.
doi: 10.7150/ijms.4276 URL |
| 21. |
Madry, H.; van Dijk, C. N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010, 18, 419-433.
doi: 10.1007/s00167-010-1054-z URL |
| 22. |
Pan, J.; Zhou, X.; Li, W.; Novotny, J. E.; Doty, S. B.; Wang, L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res. 2009, 27, 1347-1352.
doi: 10.1002/jor.20883 URL |
| 23. |
Englund, M. The role of the meniscus in osteoarthritis genesis. Rheum Dis Clin North Am. 2008, 34, 573-579.
doi: 10.1016/j.rdc.2008.05.009 URL |
| 24. |
Verdonk, P. C.; Forsyth, R. G.; Wang, J.; Almqvist, K. F.; Verdonk, R.; Veys, E. M.; Verbruggen, G. Characterisation of human knee meniscus cell phenotype. Osteoarthritis Cartilage. 2005, 13, 548-560.
doi: 10.1016/j.joca.2005.01.010 URL |
| 25. |
de Sousa, E. B.; Casado, P. L.; Moura Neto, V.; Duarte, M. E.; Aguiar, D. P. Synovial fluid and synovial membrane mesenchymal stem cells: latest discoveries and therapeutic perspectives. Stem Cell Res Ther. 2014, 5, 112.
doi: 10.1186/scrt501 URL |
| 26. |
Scanzello, C. R.; Goldring, S. R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012, 51, 249-257.
doi: 10.1016/j.bone.2012.02.012 URL |
| 27. |
Goldring, M. B.; Marcu, K. B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009, 11, 224.
doi: 10.1186/ar2592 URL |
| 28. | Sandy, J. D. A contentious issue finds some clarity: on the independent and complementary roles of aggrecanase activity and MMP activity in human joint aggrecanolysis. Osteoarthritis Cartilage. 2006, 14, 95-100. |
| 29. |
Rengel, Y.; Ospelt, C.; Gay, S. Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther. 2007, 9, 221.
doi: 10.1186/ar2304 URL |
| 30. |
Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Med Port. 2015, 28, 99-106.
doi: 10.20344/amp.5477 URL |
| 31. |
Pfander, D.; Körtje, D.; Zimmermann, R.; Weseloh, G.; Kirsch, T.; Gesslein, M.; Cramer, T.; Swoboda, B. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann Rheum Dis. 2001, 60, 1070-1073.
doi: 10.1136/ard.60.11.1070 URL |
| 32. |
Towle, C. A.; Hung, H. H.; Bonassar, L. J.; Treadwell, B. V.; Mangham, D. C. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997, 5, 293-300.
doi: 10.1016/S1063-4584(97)80008-8 URL |
| 33. |
Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; Starešinić, M.; Sabalić, S.; Dobričić, B.; Petrović, T.; Antičević, D.; Borić, I.; Košir, R.; Zmrzljak, U. P.; Primorac, D. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. 2021, 22, 9208.
doi: 10.3390/ijms22179208 URL |
| 34. | Vincenti, M. P.; Brinckerhoff, C. E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157-164. |
| 35. | Mead, T. J.; Apte, S. S. ADAMTS proteins in human disorders. Matrix Biol. 2018, 71- 72, 225-239. |
| 36. | Goldenberg, D. L.; Egan, M. S.; Cohen, A. S. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982, 9, 204-209. |
| 37. | Wood, M. J.; Leckenby, A.; Reynolds, G.; Spiering, R.; Pratt, A. G.; Rankin, K. S.; Isaacs, J. D.; Haniffa, M. A.; Milling, S.; Hilkens, C. M. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight. 2019, 4, e125325. |
| 38. |
Kraus, V. B.; McDaniel, G.; Huebner, J. L.; Stabler, T. V.; Pieper, C. F.; Shipes, S. W.; Petry, N. A.; Low, P. S.; Shen, J.; McNearney, T. A.; Mitchell, P. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016, 24, 1613-1621.
doi: 10.1016/j.joca.2016.04.010 URL |
| 39. | Muraille, E.; Leo, O.; Moser, M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014, 5, 603. |
| 40. |
Thomson, A.; Hilkens, C. M. U. Synovial macrophages in osteoarthritis: the key to understanding pathogenesis? Front Immunol. 2021, 12, 678757.
doi: 10.3389/fimmu.2021.678757 URL |
| 41. | Kurowska-Stolarska, M.; Alivernini, S. Synovial tissue macrophages: friend or foe? RMD Open. 2017, 3, e000527. |
| 42. | Ghadially, F. N.; Lalonde, J. M.; Wedge, J. H. Ultrastructure of normal and torn menisci of the human knee joint. J Anat. 1983, 136, 773-791. |
| 43. | Fuhrmann, I. K.; Steinhagen, J.; Rüther, W.; Schumacher, U. Comparative immunohistochemical evaluation of the zonal distribution of extracellular matrix and inflammation markers in human meniscus in osteoarthritis and rheumatoid arthritis. Acta Histochem. 2015, 117, 243-254. |
| 44. |
Favero, M.; Belluzzi, E.; Trisolino, G.; Goldring, M. B.; Goldring, S. R.; Cigolotti, A.; Pozzuoli, A.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; Punzi, L.; Olivotto, E. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: a coculture study. J Cell Physiol. 2019, 234, 11176-11187.
doi: 10.1002/jcp.27766 URL |
| 45. |
Gupta, T.; Zielinska, B.; McHenry, J.; Kadmiel, M.; Haut Donahue, T. L. IL-1 and iNOS gene expression and NO synthesis in the superior region of meniscal explants are dependent on the magnitude of compressive strains. Osteoarthritis Cartilage. 2008, 16, 1213-1219.
doi: 10.1016/j.joca.2008.02.019 URL |
| 46. |
DeGroot, J.; Verzijl, N.; Wenting-van Wijk, M. J.; Jacobs, K. M.; Van El, B.; Van Roermund, P. M.; Bank, R. A.; Bijlsma, J. W.; TeKoppele, J. M.; Lafeber, F. P. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004, 50, 1207-1215.
doi: 10.1002/art.20170 URL |
| 47. |
Chen, Y. J.; Chan, D. C.; Chiang, C. K.; Wang, C. C.; Yang, T. H.; Lan, K. C.; Chao, S. C.; Tsai, K. S.; Yang, R. S.; Liu, S. H. Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-κB pathway activation. J Orthop Res. 2016, 34, 791-800.
doi: 10.1002/jor.23083 URL |
| 48. |
Bonnelye, E.; Aubin, J. E. Estrogen receptor-related receptor alpha: a mediator of estrogen response in bone. J Clin Endocrinol Metab. 2005, 90, 3115-3121.
doi: 10.1210/jc.2004-2168 URL |
| 49. |
Bonnelye, E.; Vanacker, J. M.; Dittmar, T.; Begue, A.; Desbiens, X.; Denhardt, D. T.; Aubin, J. E.; Laudet, V.; Fournier, B. The ERR-1 orphan receptor is a transcriptional activator expressed during bone development. Mol Endocrinol. 1997, 11, 905-916.
doi: 10.1210/mend.11.7.9948 URL |
| 50. |
Son, Y. O.; Park, S.; Kwak, J. S.; Won, Y.; Choi, W. S.; Rhee, J.; Chun, C. H.; Ryu, J. H.; Kim, D. K.; Choi, H. S.; Chun, J. S. Estrogen-related receptor γ causes osteoarthritis by upregulating extracellular matrix-degrading enzymes. Nat Commun. 2017, 8, 2133.
doi: 10.1038/s41467-017-01868-8 URL |
| 51. |
Tang, J.; Liu, T.; Wen, X.; Zhou, Z.; Yan, J.; Gao, J.; Zuo, J. Estrogen-related receptors: novel potential regulators of osteoarthritis pathogenesis. Mol Med. 2021, 27, 5.
doi: 10.1186/s10020-021-00270-x URL |
| 52. | Conde, J.; Scotece, M.; Gómez, R.; Lopez, V.; Gómez-Reino, J. J.; Gualillo, O. Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis. 2011, 2011, 203901. |
| 53. |
Lampropoulou-Adamidou, K.; Lelovas, P.; Karadimas, E. V.; Liakou, C.; Triantafillopoulos, I. K.; Dontas, I.; Papaioannou, N. A. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2014, 24, 263-271.
doi: 10.1007/s00590-013-1205-2 URL |
| 54. |
McCoy, A. M. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol. 2015, 52, 803-818.
doi: 10.1177/0300985815588611 URL |
| 55. |
Chu, C. R.; Szczodry, M.; Bruno, S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010, 16, 105-115.
doi: 10.1089/ten.teb.2009.0452 URL |
| 56. |
Schneider-Wald, B.; von Thaden, A. K.; Schwarz, M. L. Defect models for the regeneration of articular cartilage in large animals. Orthopade. 2013, 42, 242-253.
doi: 10.1007/s00132-012-2044-2 URL |
| 57. |
Serra, C. I.; Soler, C. Animal models of osteoarthritis in small mammals. Vet Clin North Am Exot Anim Pract. 2019, 22, 211-221.
doi: 10.1016/j.cvex.2019.01.004 URL |
| 58. | Dias, I. R.; Viegas, C. A.; Carvalho, P. P. Large animal models for osteochondral regeneration. Adv Exp Med Biol. 2018, 1059, 441-501. |
| 59. |
Outerbridge, R. E. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961, 43-B, 752-757.
doi: 10.1302/0301-620X.43B4.752 URL |
| 60. | Glasson, S. S.; Chambers, M. G.; Van Den Berg, W. B.; Little, C. B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010, 18 Suppl 3, S17-23. |
| 61. | Ahern, B. J.; Parvizi, J.; Boston, R.; Schaer, T. P. Preclinical animal models in single site cartilage defect testing:a systematic review. Osteoarthritis Cartilage. 2009, 17, 705-713. |
| 62. | Wang, P.; Zhang, F.; He, Q.; Wang, J.; Shiu, H. T.; Shu, Y.; Tsang, W. P.; Liang, S.; Zhao, K.; Wan, C. Flavonoid compound icariin activates hypoxia inducible factor-1α in chondrocytes and promotes articular cartilage repair. PLoS One. 2016, 11, e0148372. |
| 63. |
Eltawil, N. M.; De Bari, C.; Achan, P.; Pitzalis, C.; Dell’accio, F. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage. 2009, 17, 695-704.
doi: 10.1016/j.joca.2008.11.003 URL |
| 64. |
Shen, K.; Liu, X.; Qin, H.; Chai, Y.; Wang, L.; Yu, B. HA-g-CS implant and moderate-intensity exercise stimulate subchondral bone remodeling and promote repair of osteochondral defects in mice. Int J Med Sci. 2021, 18, 3808-3820.
doi: 10.7150/ijms.63401 URL |
| 65. |
Cook, J. L.; Hung, C. T.; Kuroki, K.; Stoker, A. M.; Cook, C. R.; Pfeiffer, F. M.; Sherman, S. L.; Stannard, J. P. Animal models of cartilage repair. Bone Joint Res. 2014, 3, 89-94.
doi: 10.1302/2046-3758.34.2000238 URL |
| 66. |
Park, K. S.; Kim, B. J.; Lih, E.; Park, W.; Lee, S. H.; Joung, Y. K.; Han, D. K. Versatile effects of magnesium hydroxide nanoparticles in PLGA scaffold-mediated chondrogenesis. Acta Biomater. 2018, 73, 204-216.
doi: 10.1016/j.actbio.2018.04.022 URL |
| 67. | Gregory, M. H.; Capito, N.; Kuroki, K.; Stoker, A. M.; Cook, J. L.; Sherman, S. L. A review of translational animal models for knee osteoarthritis. Arthritis. 2012, 2012, 764621. |
| 68. |
Proffen, B. L.; McElfresh, M.; Fleming, B. C.; Murray, M. M. A comparative anatomical study of the human knee and six animal species. Knee. 2012, 19, 493-499.
doi: 10.1016/j.knee.2011.07.005 URL |
| 69. |
Räsänen, T.; Messner, K. Regional variations of indentation stiffness and thickness of normal rabbit knee articular cartilage. J Biomed Mater Res. 1996, 31, 519-524.
doi: 10.1002/(SICI)1097-4636(199608)31:4<519::AID-JBM12>3.0.CO;2-B URL |
| 70. |
Radhakrishnan, J.; Manigandan, A.; Chinnaswamy, P.; Subramanian, A.; Sethuraman, S. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials. 2018, 162, 82-98.
doi: 10.1016/j.biomaterials.2018.01.056 URL |
| 71. |
Levingstone, T. J.; Thompson, E.; Matsiko, A.; Schepens, A.; Gleeson, J. P.; O’Brien, F. J. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 2016, 32, 149-160.
doi: 10.1016/j.actbio.2015.12.034 URL |
| 72. | Ramallal, M.; Maneiro, E.; López, E.; Fuentes-Boquete, I.; López-Armada, M. J.; Fernández-Sueiro, J. L.; Galdo, F.; De Toro, F. J.; Blanco, F. J. Xeno-implantation of pig chondrocytes into rabbit to treat localized articular cartilage defects: an animal model. Wound Repair Regen. 2004, 12, 337-345. |
| 73. | Cook, J. L.; Kuroki, K.; Visco, D.; Pelletier, J. P.; Schulz, L.; Lafeber, F. P. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the dog. Osteoarthritis Cartilage. 2010, 18 Suppl 3, S66-79. |
| 74. |
Shortkroff, S.; Barone, L.; Hsu, H. P.; Wrenn, C.; Gagne, T.; Chi, T.; Breinan, H.; Minas, T.; Sledge, C. B.; Tubo, R.; Spector, M. Healing of chondral and osteochondral defects in a canine model: the role of cultured chondrocytes in regeneration of articular cartilage. Biomaterials. 1996, 17, 147-154.
doi: 10.1016/0142-9612(96)85759-0 URL |
| 75. |
van Dyk, G. E.; Dejardin, L. M.; Flo, G.; Johnson, L. L. Cancellous bone grafting of large osteochondral defects: an experimental study in dogs. Arthroscopy. 1998, 14, 311-320.
doi: 10.1016/S0749-8063(98)70148-3 URL |
| 76. | Cook, S. D.; Patron, L. P.; Salkeld, S. L.; Rueger, D. C. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg Am. 2003, 85-A Suppl 3, 116-123. |
| 77. |
Kazemi, D.; Shams Asenjan, K.; Dehdilani, N.; Parsa, H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: macroscopic and histological assessments. Bone Joint Res. 2017, 6, 98-107.
doi: 10.1302/2046-3758.62.BJR-2016-0188.R1 URL |
| 78. |
Vernon, L.; Abadin, A.; Wilensky, D.; Huang, C. Y.; Kaplan, L. Subphysiological compressive loading reduces apoptosis following acute impact injury in a porcine cartilage model. Sports Health. 2014, 6, 81-88.
doi: 10.1177/1941738113504379 URL |
| 79. |
Frisbie, D. D.; Cross, M. W.; McIlwraith, C. W. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006, 19, 142-146.
doi: 10.1055/s-0038-1632990 URL |
| 80. | Gotterbarm, T.; Breusch, S. J.; Schneider, U.; Jung, M. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim. 2008, 42, 71-82. |
| 81. |
Vasara, A. I.; Hyttinen, M. M.; Pulliainen, O.; Lammi, M. J.; Jurvelin, J. S.; Peterson, L.; Lindahl, A.; Helminen, H. J.; Kiviranta, I. Immature porcine knee cartilage lesions show good healing with or without autologous chondrocyte transplantation. Osteoarthritis Cartilage. 2006, 14, 1066-1074.
doi: 10.1016/j.joca.2006.04.003 URL |
| 82. | Hembry, R. M.; Dyce, J.; Driesang, I.; Hunziker, E. B.; Fosang, A. J.; Tyler, J. A.; Murphy, G. Immunolocalization of matrix metalloproteinases in partial-thickness defects in pig articular cartilage. A preliminary report. J Bone Joint Surg Am. 2001, 83, 826-838. |
| 83. |
Chang, C. H.; Kuo, T. F.; Lin, C. C.; Chou, C. H.; Chen, K. H.; Lin, F. H.; Liu, H. C. Tissue engineering-based cartilage repair with allogenous chondrocytes and gelatin-chondroitin-hyaluronan tri-copolymer scaffold: a porcine model assessed at 18, 24, and 36 weeks. Biomaterials. 2006, 27, 1876-1888.
doi: 10.1016/j.biomaterials.2005.10.014 URL |
| 84. |
Lu, Y.; Hayashi, K.; Hecht, P.; Fanton, G. S.; Thabit, G., 3rd; Cooley, A. J.; Edwards, R. B.; Markel, M. D. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000, 16, 527-536.
doi: 10.1053/jars.2000.7690 URL |
| 85. |
Tytherleigh-Strong, G.; Hurtig, M.; Miniaci, A. Intra-articular hyaluronan following autogenous osteochondral grafting of the knee. Arthroscopy. 2005, 21, 999-1005.
doi: 10.1016/j.arthro.2005.05.001 URL |
| 86. |
Siebert, C. H.; Miltner, O.; Weber, M.; Sopka, S.; Koch, S.; Niedhart, C. Healing of osteochondral grafts in an ovine model under the influence of bFGF. Arthroscopy. 2003, 19, 182-187.
doi: 10.1053/jars.2003.50000 URL |
| 87. |
Kandel, R. A.; Grynpas, M.; Pilliar, R.; Lee, J.; Wang, J.; Waldman, S.; Zalzal, P.; Hurtig, M. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials. 2006, 27, 4120-4131.
doi: 10.1016/j.biomaterials.2006.03.005 URL |
| 88. |
Mohan, N.; Gupta, V.; Sridharan, B. P.; Mellott, A. J.; Easley, J. T.; Palmer, R. H.; Galbraith, R. A.; Key, V. H.; Berkland, C. J.; Detamore, M. S. Microsphere-based gradient implants for osteochondral regeneration: a long-term study in sheep. Regen Med. 2015, 10, 709-728.
doi: 10.2217/rme.15.38 URL |
| 89. |
Brehm, W.; Aklin, B.; Yamashita, T.; Rieser, F.; Trüb, T.; Jakob, R. P.; Mainil-Varlet, P. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006, 14, 1214-1226.
doi: 10.1016/j.joca.2006.05.002 URL |
| 90. |
Jackson, D. W.; Lalor, P. A.; Aberman, H. M.; Simon, T. M. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg Am. 2001, 83, 53-64.
doi: 10.2106/00004623-200101000-00008 URL |
| 91. |
Niederauer, G. G.; Slivka, M. A.; Leatherbury, N. C.; Korvick, D. L.; Harroff, H. H.; Ehler, W. C.; Dunn, C. J.; Kieswetter, K. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000, 21, 2561-2574.
doi: 10.1016/S0142-9612(00)00124-1 URL |
| 92. |
Dell’Accio, F.; Vanlauwe, J.; Bellemans, J.; Neys, J.; De Bari, C.; Luyten, F. P. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res. 2003, 21, 123-131.
doi: 10.1016/S0736-0266(02)00090-6 URL |
| 93. |
Kon, E.; Robinson, D.; Shani, J.; Alves, A.; Di Matteo, B.; Ashmore, K.; De Caro, F.; Dulic, O.; Altschuler, N. Reconstruction of large osteochondral defects using a hemicondylar aragonite-based implant in a caprine model. Arthroscopy. 2020, 36, 1884-1894.
doi: 10.1016/j.arthro.2020.02.026 URL |
| 94. |
Malda, J.; Benders, K. E.; Klein, T. J.; de Grauw, J. C.; Kik, M. J.; Hutmacher, D. W.; Saris, D. B.; van Weeren, P. R.; Dhert, W. J. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthritis Cartilage. 2012, 20, 1147-1151.
doi: 10.1016/j.joca.2012.06.005 URL |
| 95. | Convery, F. R.; Akeson, W. H.; Keown, G. H. The repair of large osteochondral defects. An experimental study in horses. Clin Orthop Relat Res. 1972, 82, 253-262. |
| 96. |
Hidaka, C.; Goodrich, L. R.; Chen, C. T.; Warren, R. F.; Crystal, R. G.; Nixon, A. J. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003, 21, 573-583.
doi: 10.1016/S0736-0266(02)00264-4 URL |
| 97. |
Strauss, E. J.; Goodrich, L. R.; Chen, C. T.; Hidaka, C.; Nixon, A. J. Biochemical and biomechanical properties of lesion and adjacent articular cartilage after chondral defect repair in an equine model. Am J Sports Med. 2005, 33, 1647-1653.
doi: 10.1177/0363546505275487 URL |
| 98. |
Seo, J. P.; Kambayashi, Y.; Itho, M.; Haneda, S.; Yamada, K.; Furuoka, H.; Tabata, Y.; Sasaki, N. Effects of a synovial flap and gelatin/β-tricalcium phosphate sponges loaded with mesenchymal stem cells, bone morphogenetic protein-2, and platelet rich plasma on equine osteochondral defects. Res Vet Sci. 2015, 101, 140-143.
doi: 10.1016/j.rvsc.2015.06.014 URL |
| 99. |
Vindas Bolaños, R. A.; Cokelaere, S. M.; Estrada McDermott, J. M.; Benders, K. E.; Gbureck, U.; Plomp, S. G.; Weinans, H.; Groll, J.; van Weeren, P. R.; Malda, J. The use of a cartilage decellularized matrix scaffold for the repair of osteochondral defects: the importance of long-term studies in a large animal model. Osteoarthritis Cartilage. 2017, 25, 413-420.
doi: 10.1016/j.joca.2016.08.005 URL |
| 100. |
McCarrel, T. M.; Pownder, S. L.; Gilbert, S.; Koff, M. F.; Castiglione, E.; Saska, R. A.; Bradica, G.; Fortier, L. A. Two-year evaluation of osteochondral repair with a novel biphasic graft saturated in bone marrow in an equine model. Cartilage. 2017, 8, 406-416.
doi: 10.1177/1947603516675913 URL |
| 101. | Maninchedda, U.; Lepage, O. M.; Gangl, M.; Hilairet, S.; Remandet, B.; Meot, F.; Penarier, G.; Segard, E.; Cortez, P.; Jorgensen, C.; Steinberg, R. Development of an equine groove model to induce metacarpophalangeal osteoarthritis: a pilot study on 6 horses. PLoS One. 2015, 10, e0115089. |
| 102. |
Carlson, C. S.; Loeser, R. F.; Jayo, M. J.; Weaver, D. S.; Adams, M. R.; Jerome, C. P. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994, 12, 331-339.
doi: 10.1002/jor.1100120305 URL |
| 103. |
Macrini, T. E.; Coan, H. B.; Levine, S. M.; Lerma, T.; Saks, C. D.; Araujo, D. J.; Bredbenner, T. L.; Coutts, R. D.; Nicolella, D. P.; Havill, L. M. Reproductive status and sex show strong effects on knee OA in a baboon model. Osteoarthritis Cartilage. 2013, 21, 839-848.
doi: 10.1016/j.joca.2013.03.003 URL |
| 104. |
Carlson, C. S.; Loeser, R. F.; Purser, C. B.; Gardin, J. F.; Jerome, C. P. Osteoarthritis in cynomolgus macaques. III: Effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res. 1996, 11, 1209-1217.
doi: 10.1002/jbmr.5650110904 URL |
| 105. |
Black, A.; Lane, M. A. Nonhuman primate models of skeletal and reproductive aging. Gerontology. 2002, 48, 72-80.
doi: 10.1159/000048930 URL |
| 106. | Buckwalter, J. A.; Martin, J. A.; Olmstead, M.; Athanasiou, K. A.; Rosenwasser, M. P.; Mow, V. C. Osteochondral repair of primate knee femoral and patellar articular surfaces: implications for preventing post-traumatic osteoarthritis. Iowa Orthop J. 2003, 23, 66-74. |
| 107. | Zhou, L.; Gjvm, V. O.; Malda, J.; Stoddart, M. J.; Lai, Y.; Richards, R. G.; Ki-Wai Ho, K.; Qin, L. Innovative tissue-engineered strategies for osteochondral defect repair and regeneration: current progress and challenges. Adv Healthc Mater. 2020, e2001008. |
| 108. |
Wesdorp, M. A.; Capar, S.; Bastiaansen-Jenniskens, Y. M.; Kops, N.; Creemers, L. B.; Verhaar, J. A. N.; Van Osch, G.; Wei, W. Intra-articular administration of triamcinolone acetonide in a murine cartilage defect model reduces inflammation but inhibits endogenous cartilage repair. Am J Sports Med. 2022, 50, 1668-1678.
doi: 10.1177/03635465221083693 URL |
| 109. |
Marycz, K.; Smieszek, A.; Targonska, S.; Walsh, S. A.; Szustakiewicz, K.; Wiglusz, R. J. Three dimensional (3D) printed polylactic acid with nano-hydroxyapatite doped with europium(III) ions (nHAp/PLLA@Eu(3+)) composite for osteochondral defect regeneration and theranostics. Mater Sci Eng C Mater Biol Appl. 2020, 110, 110634.
doi: 10.1016/j.msec.2020.110634 URL |
| 110. |
Mendes, L. F.; Katagiri, H.; Tam, W. L.; Chai, Y. C.; Geris, L.; Roberts, S. J.; Luyten, F. P. Advancing osteochondral tissue engineering: bone morphogenetic protein, transforming growth factor, and fibroblast growth factor signaling drive ordered differentiation of periosteal cells resulting in stable cartilage and bone formation in vivo. Stem Cell Res Ther. 2018, 9, 42.
doi: 10.1186/s13287-018-0787-3 URL |
| 111. |
Li, X.; Guo, W.; Zha, K.; Jing, X.; Wang, M.; Zhang, Y.; Hao, C.; Gao, S.; Chen, M.; Yuan, Z.; Wang, Z.; Zhang, X.; Shen, S.; Li, H.; Zhang, B.; Xian, H.; Zhang, Y.; Sui, X.; Qin, L.; Peng, J.; Liu, S.; Lu, S.; Guo, Q. Enrichment of CD146(+) adipose-derived stem cells in combination with articular cartilage extracellular matrix scaffold promotes cartilage regeneration. Theranostics. 2019, 9, 5105-5121.
doi: 10.7150/thno.33904 URL |
| 112. |
Ji, X.; Shao, H.; Li, X.; Ullah, M. W.; Luo, G.; Xu, Z.; Ma, L.; He, X.; Lei, Z.; Li, Q.; Jiang, X.; Yang, G.; Zhang, Y. Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration. Biomaterials. 2022, 285, 121530.
doi: 10.1016/j.biomaterials.2022.121530 URL |
| 113. |
Kim, H. S.; Mandakhbayar, N.; Kim, H. W.; Leong, K. W.; Yoo, H. S. Protein-reactive nanofibrils decorated with cartilage-derived decellularized extracellular matrix for osteochondral defects. Biomaterials. 2021, 269, 120214.
doi: 10.1016/j.biomaterials.2020.120214 URL |
| 114. |
Lin, D.; Cai, B.; Wang, L.; Cai, L.; Wang, Z.; Xie, J.; Lv, Q. X.; Yuan, Y.; Liu, C.; Shen, S. G. A viscoelastic PEGylated poly(glycerol sebacate)-based bilayer scaffold for cartilage regeneration in full-thickness osteochondral defect. Biomaterials. 2020, 253, 120095.
doi: 10.1016/j.biomaterials.2020.120095 URL |
| 115. | Yang, M.; Zhang, Z. C.; Yuan, F. Z.; Deng, R. H.; Yan, X.; Mao, F. B.; Chen, Y. R.; Lu, H.; Yu, J. K. An immunomodulatory polypeptide hydrogel for osteochondral defect repair. Bioact Mater. 2023, 19, 678-689. |
| 116. |
Qi, Y.; Zhang, W.; Li, G.; Niu, L.; Zhang, Y.; Tang, R.; Feng, G. An oriented-collagen scaffold including Wnt5a promotes osteochondral regeneration and cartilage interface integration in a rabbit model. FASEB J. 2020, 34, 11115-11132.
doi: 10.1096/fj.202000280R URL |
| 117. |
Ye, W.; Yang, Z.; Cao, F.; Li, H.; Zhao, T.; Zhang, H.; Zhang, Z.; Yang, S.; Zhu, J.; Liu, Z.; Zheng, J.; Liu, H.; Ma, G.; Guo, Q.; Wang, X. Articular cartilage reconstruction with TGF-β1-simulating self-assembling peptide hydrogel-based composite scaffold. Acta Biomater. 2022, 146, 94-106.
doi: 10.1016/j.actbio.2022.05.012 URL |
| 118. | Wu, H.; Shang, Y.; Sun, W.; Ouyang, X.; Zhou, W.; Lu, J.; Yang, S.; Wei, W.; Yao, X.; Wang, X.; Zhang, X.; Chen, Y.; He, Q.; Yang, Z.; Ouyang, H. Seamless and early gap healing of osteochondral defects by autologous mosaicplasty combined with bioactive supramolecular nanofiber-enabled gelatin methacryloyl (BSN-GelMA) hydrogel. Bioact Mater. 2023, 19, 88-102. |
| 119. |
Hong, Y.; Han, Y.; Wu, J.; Zhao, X.; Cheng, J.; Gao, G.; Qian, Q.; Wang, X.; Cai, W.; Zreiqat, H.; Feng, D.; Xu, J.; Cui, D. Chitosan modified Fe(3)O(4)/KGN self-assembled nanoprobes for osteochondral MR diagnose and regeneration. Theranostics. 2020, 10, 5565-5577.
doi: 10.7150/thno.43569 URL |
| 120. |
Sun, J.; Lyu, J.; Xing, F.; Chen, R.; Duan, X.; Xiang, Z. A biphasic, demineralized, and Decellularized allograft bone-hydrogel scaffold with a cell-based BMP-7 delivery system for osteochondral defect regeneration. J Biomed Mater Res A. 2020, 108, 1909-1921.
doi: 10.1002/jbm.a.36954 URL |
| 121. |
Baba, R.; Onodera, T.; Matsuoka, M.; Hontani, K.; Joutoku, Z.; Matsubara, S.; Homan, K.; Iwasaki, N. Bone marrow stimulation technique augmented by an ultrapurified alginate gel enhances cartilage repair in a canine model. Am J Sports Med. 2018, 46, 1970-1979.
doi: 10.1177/0363546518770436 URL |
| 122. | Onodera, T.; Baba, R.; Kasahara, Y.; Tsuda, T.; Iwasaki, N. Therapeutic effects and adaptive limits of an acellular technique by ultrapurified alginate (UPAL) gel implantation in canine osteochondral defect models. Regen Ther. 2020, 14, 154-159. |
| 123. |
Ryu, J.; Brittberg, M.; Nam, B.; Chae, J.; Kim, M.; Colon Iban, Y.; Magneli, M.; Takahashi, E.; Khurana, B.; Bragdon, C. R. Evaluation of three-dimensional bioprinted human cartilage powder combined with micronized subcutaneous adipose tissues for the repair of osteochondral defects in beagle dogs. Int J Mol Sci. 2022, 23, 2743.
doi: 10.3390/ijms23052743 URL |
| 124. |
Stefani, R. M.; Lee, A. J.; Tan, A. R.; Halder, S. S.; Hu, Y.; Guo, X. E.; Stoker, A. M.; Ateshian, G. A.; Marra, K. G.; Cook, J. L.; Hung, C. T. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020, 102, 326-340.
doi: 10.1016/j.actbio.2019.11.052 URL |
| 125. |
Yan, W.; Xu, X.; Xu, Q.; Sun, Z.; Lv, Z.; Wu, R.; Yan, W.; Jiang, Q.; Shi, D. An injectable hydrogel scaffold with kartogenin-encapsulated nanoparticles for porcine cartilage regeneration: a 12-month follow-up study. Am J Sports Med. 2020, 48, 3233-3244.
doi: 10.1177/0363546520957346 URL |
| 126. |
Lin, C. C.; Chu, C. J.; Chou, P. H.; Liang, C. H.; Liang, P. I.; Chang, N. J. Beneficial therapeutic approach of acellular PLGA implants coupled with rehabilitation exercise for osteochondral repair: a proof of concept study in a minipig model. Am J Sports Med. 2020, 48, 2796-2807.
doi: 10.1177/0363546520940306 URL |
| 127. |
Steele, J. A. M.; Moore, A. C.; St-Pierre, J. P.; McCullen, S. D.; Gormley, A. J.; Horgan, C. C.; Black, C. R.; Meinert, C.; Klein, T.; Saifzadeh, S.; Steck, R.; Ren, J.; Woodruff, M. A.; Stevens, M. M. In vitro and in vivo investigation of a zonal microstructured scaffold for osteochondral defect repair. Biomaterials. 2022, 286, 121548.
doi: 10.1016/j.biomaterials.2022.121548 URL |
| 128. |
Asen, A. K.; Goebel, L.; Rey-Rico, A.; Sohier, J.; Zurakowski, D.; Cucchiarini, M.; Madry, H. Sustained spatiotemporal release of TGF-β1 confers enhanced very early chondrogenic differentiation during osteochondral repair in specific topographic patterns. FASEB J. 2018, 32, 5298-5311.
doi: 10.1096/fj.201800105R URL |
| 129. |
Huang, Y.; Fan, H.; Gong, X.; Yang, L.; Wang, F. Scaffold with natural calcified cartilage zone for osteochondral defect repair in minipigs. Am J Sports Med. 2021, 49, 1883-1891.
doi: 10.1177/03635465211007139 URL |
| 130. |
Bozkurt, M.; Aşık, M. D.; Gürsoy, S.; Türk, M.; Karahan, S.; Gümüşkaya, B.; Akkaya, M.; Şimşek, M. E.; Cay, N.; Doğan, M. Autologous stem cell-derived chondrocyte implantation with bio-targeted microspheres for the treatment of osteochondral defects. J Orthop Surg Res. 2019, 14, 394.
doi: 10.1186/s13018-019-1434-0 URL |
| 131. | Vukasovic, A.; Asnaghi, M. A.; Kostesic, P.; Quasnichka, H.; Cozzolino, C.; Pusic, M.; Hails, L.; Trainor, N.; Krause, C.; Figallo, E.; Filardo, G.; Kon, E.; Wixmerten, A.; Maticic, D.; Pellegrini, G.; Kafienah, W.; Hudetz, D.; Smith, T.; Martin, I.; Ivkovic, A.; Wendt, D. Bioreactor-manufactured cartilage grafts repair acute and chronic osteochondral defects in large animal studies. Cell Prolif. 2019, 52, e12653. |
| 132. | Tamaddon, M.; Blunn, G.; Tan, R.; Yang, P.; Sun, X.; Chen, S. M.; Luo, J.; Liu, Z.; Wang, L.; Li, D.; Donate, R.; Monzón, M.; Liu, C. In vivo evaluation of additively manufactured multi-layered scaffold for the repair of large osteochondral defects. Biodes Manuf. 2022, 5, 481-496. |
| 133. | Favreau, H.; Pijnenburg, L.; Seitlinger, J.; Fioretti, F.; Keller, L.; Scipioni, D.; Adriaensen, H.; Kuchler-Bopp, S.; Ehlinger, M.; Mainard, D.; Rosset, P.; Hua, G.; Gentile, L.; Benkirane-Jessel, N. Osteochondral repair combining therapeutics implant with mesenchymal stem cells spheroids. Nanomedicine. 2020, 29, 102253. |
| 134. |
Critchley, S.; Sheehy, E. J.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S. F.; Jeon, O.; Alsberg, E.; Brama, P. A. J.; Kelly, D. J. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020, 113, 130-143.
doi: 10.1016/j.actbio.2020.05.040 URL |
| 135. |
Jia, S.; Wang, J.; Zhang, T.; Pan, W.; Li, Z.; He, X.; Yang, C.; Wu, Q.; Sun, W.; Xiong, Z.; Hao, D. Multilayered scaffold with a compact interfacial layer enhances osteochondral defect repair. ACS Appl Mater Interfaces. 2018, 10, 20296-20305.
doi: 10.1021/acsami.8b03445 URL |
| 136. |
Burdis, R.; Chariyev-Prinz, F.; Browe, D. C.; Freeman, F. E.; Nulty, J.; McDonnell, E. E.; Eichholz, K. F.; Wang, B.; Brama, P.; Kelly, D. J. Spatial patterning of phenotypically distinct microtissues to engineer osteochondral grafts for biological joint resurfacing. Biomaterials. 2022, 289, 121750.
doi: 10.1016/j.biomaterials.2022.121750 URL |
| 137. |
Cunniffe, G. M.; Díaz-Payno, P. J.; Sheehy, E. J.; Critchley, S. E.; Almeida, H. V.; Pitacco, P.; Carroll, S. F.; Mahon, O. R.; Dunne, A.; Levingstone, T. J.; Moran, C. J.; Brady, R. T.; O’Brien, F. J.; Brama, P. A. J.; Kelly, D. J. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials. 2019, 188, 63-73.
doi: 10.1016/j.biomaterials.2018.09.044 URL |
| 138. |
Korthagen, N. M.; Brommer, H.; Hermsen, G.; Plomp, S. G. M.; Melsom, G.; Coeleveld, K.; Mastbergen, S. C.; Weinans, H.; van Buul, W.; van Weeren, P. R. A short-term evaluation of a thermoplastic polyurethane implant for osteochondral defect repair in an equine model. Vet J. 2019, 251, 105340.
doi: 10.1016/j.tvjl.2019.105340 URL |
| 139. |
Mancini, I. A. D.; Schmidt, S.; Brommer, H.; Pouran, B.; Schäfer, S.; Tessmar, J.; Mensinga, A.; van Rijen, M. H. P.; Groll, J.; Blunk, T.; Levato, R.; Malda, J.; van Weeren, P. R. A composite hydrogel-3D printed thermoplast osteochondral anchor as example for a zonal approach to cartilage repair: in vivo performance in a long-term equine model. Biofabrication. 2020, 12, 035028.
doi: 10.1088/1758-5090/ab94ce URL |
| 140. |
Murata, D.; Ishikawa, S.; Sunaga, T.; Saito, Y.; Sogawa, T.; Nakayama, K.; Hobo, S.; Hatazoe, T. Osteochondral regeneration of the femoral medial condyle by using a scaffold-free 3D construct of synovial membrane-derived mesenchymal stem cells in horses. BMC Vet Res. 2022, 18, 53.
doi: 10.1186/s12917-021-03126-y URL |
| 141. |
Zanotto, G. M.; Liesbeny, P.; Barrett, M.; Zlotnick, H.; Frank, E.; Grodzinsky, A. J.; Frisbie, D. D. Microfracture augmentation with trypsin pretreatment and growth factor-functionalized self-assembling peptide hydrogel scaffold in an equine model. Am J Sports Med. 2021, 49, 2498-2508.
doi: 10.1177/03635465211021798 URL |
| 142. |
Park, D. Y.; Min, B. H.; Park, S. R.; Oh, H. J.; Truong, M. D.; Kim, M.; Choi, J. Y.; Park, I. S.; Choi, B. H. Engineered cartilage utilizing fetal cartilage-derived progenitor cells for cartilage repair. Sci Rep. 2020, 10, 5722.
doi: 10.1038/s41598-020-62580-0 URL |
| 143. |
Jiang, L.; Ma, A.; Song, L.; Hu, Y.; Dun, H.; Daloze, P.; Yu, Y.; Jiang, J.; Zafarullah, M.; Chen, H. Cartilage regeneration by selected chondrogenic clonal mesenchymal stem cells in the collagenase-induced monkey osteoarthritis model. J Tissue Eng Regen Med. 2014, 8, 896-905.
doi: 10.1002/term.1676 URL |
| 144. |
Ma, A.; Jiang, L.; Song, L.; Hu, Y.; Dun, H.; Daloze, P.; Yu, Y.; Jiang, J.; Zafarullah, M.; Chen, H. Reconstruction of cartilage with clonal mesenchymal stem cell-acellular dermal matrix in cartilage defect model in nonhuman primates. Int Immunopharmacol. 2013, 16, 399-408.
doi: 10.1016/j.intimp.2013.02.005 URL |
| 145. | Maglio, M.; Brogini, S.; Pagani, S.; Giavaresi, G.; Tschon, M. Current trends in the evaluation of osteochondral lesion treatments: histology, histomorphometry, and biomechanics in preclinical models. Biomed Res Int. 2019, 2019, 4040236. |
| 146. |
Yin, H.; Wang, Y.; Sun, X.; Cui, G.; Sun, Z.; Chen, P.; Xu, Y.; Yuan, X.; Meng, H.; Xu, W.; Wang, A.; Guo, Q.; Lu, S.; Peng, J. Functional tissue-engineered microtissue derived from cartilage extracellular matrix for articular cartilage regeneration. Acta Biomater. 2018, 77, 127-141.
doi: 10.1016/j.actbio.2018.07.031 URL |
| 147. |
Martin-Hernandez, C.; Cebamanos-Celma, J.; Molina-Ros, A.; Ballester-Jimenez, J. J.; Ballester-Soleda, J. Regenerated cartilage produced by autogenous periosteal grafts: a histologic and mechanical study in rabbits under the influence of continuous passive motion. Arthroscopy. 2010, 26, 76-83.
doi: 10.1016/j.arthro.2009.07.005 URL |
| 148. |
Filardo, G.; Perdisa, F.; Gelinsky, M.; Despang, F.; Fini, M.; Marcacci, M.; Parrilli, A. P.; Roffi, A.; Salamanna, F.; Sartori, M.; Schütz, K.; Kon, E. Novel alginate biphasic scaffold for osteochondral regeneration: an in vivo evaluation in rabbit and sheep models. J Mater Sci Mater Med. 2018, 29, 74.
doi: 10.1007/s10856-018-6074-0 URL |
| 149. | Jansen, E. J.; Pieper, J.; Gijbels, M. J.; Guldemond, N. A.; Riesle, J.; Van Rhijn, L. W.; Bulstra, S. K.; Kuijer, R. PEOT/PBT based scaffolds with low mechanical properties improve cartilage repair tissue formation in osteochondral defects. J Biomed Mater Res A. 2009, 89, 444-452. |
| [1] | Jingyu Fan, Elizabeth Pung, Yuan Lin, Qian Wang. Recent development of hydrogen sulfide-releasing biomaterials as novel therapies:a narrative review [J]. Biomaterials Translational, 2022, 3(4): 250-263. |
| [2] | Yiqiang Hu, Yuan Xiong, Ranyang Tao, Hang Xue, Lang Chen, Ze Lin, Adriana C. Panayi, Bobin Mi, Guohui Liu. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing [J]. Biomaterials Translational, 2022, 3(3): 188-200. |
| [3] | Shuqin Cao, Quan Yuan. An update of nanotopographical surfaces in modulating stem cell fate: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 55-64. |
| [4] | Emma Steijvers, Armaan Ghei, Zhidao Xia. Manufacturing artificial bone allografts: a perspective [J]. Biomaterials Translational, 2022, 3(1): 65-80. |
| [5] | Ke Hu, Yuxuan Li, Zunxiang Ke, Hongjun Yang, Chanjun Lu, Yiqing Li, Yi Guo, Weici Wang. History, progress and future challenges of artificial blood vessels: a narrative review [J]. Biomaterials Translational, 2022, 3(1): 81-98. |
| [6] | Yizhong Peng, Jinye Li, Hui Lin, Shuo Tian, Sheng Liu, Feifei Pu, Lei Zhao, Kaige Ma, Xiangcheng Qing, Zengwu Shao. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 343-360. |
| [7] | Ge Yan, Yuqi Liu, Minghui Xie, Jiawei Shi, Weihua Qiao, Nianguo Dong. Experimental and computational models for tissue-engineered heart valves: a narrative review [J]. Biomaterials Translational, 2021, 2(4): 361-375. |
| [8] | Xirui Jing, Qiuyue Ding, Qinxue Wu, Weijie Su, Keda Yu, Yanlin Su, Bing Ye, Qing Gao, Tingfang Sun, Xiaodong Guo. Magnesium-based materials in orthopaedics: material properties and animal models [J]. Biomaterials Translational, 2021, 2(3): 197-213. |
| [9] | Aditya Joshi, George Dias, Mark P. Staiger. In silico modelling of the corrosion of biodegradable magnesium-based biomaterials: modelling approaches, validation and future perspectives [J]. Biomaterials Translational, 2021, 2(3): 257-271. |
| [10] | Kamolrat Metavarayuth, Esteban Villarreal, Hui Wang, Qian Wang. Surface topography and free energy regulate osteogenesis of stem cells: effects of shape-controlled gold nanoparticles [J]. Biomaterials Translational, 2021, 2(2): 165-173. |
| [11] | Yizhong Peng, Xiangcheng Qing, Hongyang Shu, Shuo Tian, Wenbo Yang, Songfeng Chen, Hui Lin, Xiao Lv, Lei Zhao, Xi Chen, Feifei Pu, Donghua Huang, Xu Cao, Zengwu Shao. Proper animal experimental designs for preclinical research of biomaterials for intervertebral disc regeneration [J]. Biomaterials Translational, 2021, 2(2): 91-142. |
| [12] | Pingli Wu, Yangyang Liang, Guoming Sun. Engineering immune-responsive biomaterials for skin regeneration [J]. Biomaterials Translational, 2021, 2(1): 61-71. |
| [13] | Yiqing Wang, Xiangyu Chu, Bing Wang. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine [J]. Biomaterials Translational, 2021, 2(1): 19-29. |
| [14] | Isak Jatoi, Jingyu Fan. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development [J]. Biomaterials Translational, 2021, 2(1): 30-42. |
| [15] | Jishan Yuan, Panita Maturavongsadit, Zhihui Zhou, Bin Lv, Yuan Lin, Jia Yang, Jittima Amie Luckanagul. Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats [J]. Biomaterials Translational, 2020, 1(1): 89-98. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||